|
Case Report
A durable response to gemcitabine monotherapy in metastatic cardiac angiosarcoma
1 House Officer, Department of Internal Medicine, Henry Ford Health, 2799 West Grand Blvd, Detroit, MI 48202, United States
2 House Officer, Department of Pathology, Henry Ford Health, 2799 West Grand Blvd, Detroit, MI 48202, United States
3 Senior Staff Physician, Department of Hematology/Oncology, Henry Ford Cancer Institute, 2800 West Grand Blvd, Detroit, MI 48202, United States
Address correspondence to:
Matthew Meranda
Department of Internal Medicine, Henry Ford Health, 2799 West Grand Blvd, Detroit, MI 48202,
United States
Message to Corresponding Author
Article ID: 100132Z10MM2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Meranda M, Gokturk-Ozcan G, Girgis M. A durable response to gemcitabine monotherapy in metastatic cardiac angiosarcoma. J Case Rep Images Oncology 2024;10(1):27–30.ABSTRACT
Introduction: Primary cardiac angiosarcoma is a rare neoplasm with high rates of local recurrence and distant metastasis for which optimum treatment is poorly defined.
Case Report: We present the case of a 49-year-old man with cardiac angiosarcoma with distant metastases who exhibits durable response to gemcitabine monotherapy. At initial diagnosis, he underwent complete resection with adjuvant Adriamycin and Ifosfamide for four cycles as well as external beam radiation for possible residual disease. He had recurrence with liver and bone metastases four years later. He received Gemcitabine and Docetaxel for six cycles followed by Gemcitabine monotherapy with no evidence of recurrence for the next three years.
Conclusion: This patient’s consistent response to gemcitabine maintenance therapy in metastatic cardiac angiosarcoma adds to relative paucity of data regarding the management of this rare malignancy, offering insight into best practice and hope for patients afflicted with this disease.
Keywords: Cardiac angiosarcoma, Cardio-oncology, Gemcitabine
Introduction
Primary cardiac angiosarcoma is a rare clinical entity which carries a poor prognosis due to its aggressive nature and high rates of local recurrence and distant metastasis. Though there is little consensus on optimal management, current approaches center on surgical resection with or without neoadjuvant chemo- or radiotherapy followed by adjuvant chemotherapeutics including paclitaxel, docetaxel, doxorubicin, ifosfamide, or gemcitabine. Here, we present the case of a young man who has demonstrated durable response to gemcitabine monotherapy following metastatic progression of primary cardiac angiosarcoma.
Case Report
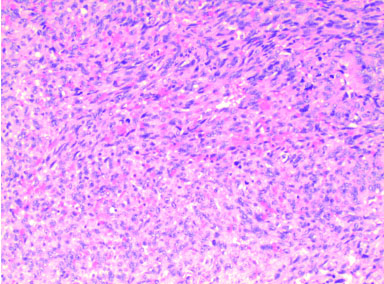
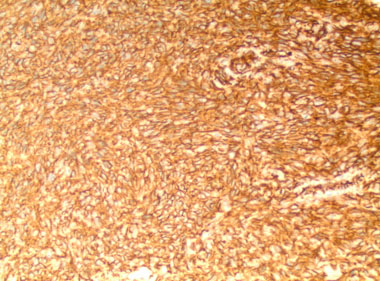
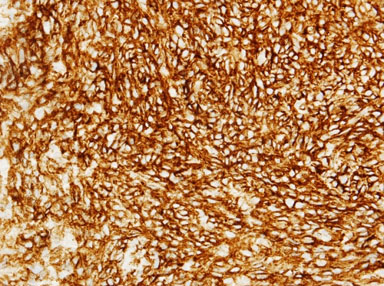
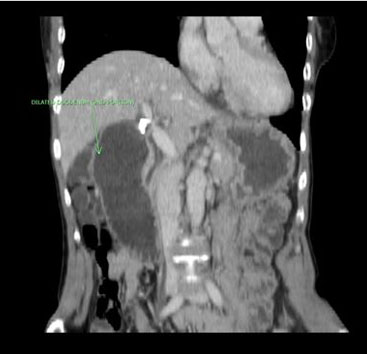
We present the case of a 49-year-old man who initially presented to hospital with chest pain, diffuse ST segment changes on electrocardiogram, and elevated cardiac biomarkers. Left heart catheterization was without evidence of coronary disease and an echocardiogram demonstrated a right atrial mass with preserved left ventricular ejection fraction of 63%. Subsequent cardiac magnetic resonance imaging (MRI) confirmed the findings of a 6.7×8.4×4.7 cm infiltrative mass involving the right atrial free wall with possible pericardial extension. Histomorphological features of the resected mass were ultimately consistent with primary cardiac angiosarcoma, demonstrating proliferation of spindle cells with marked pleomorphism (Figure 1), along with numerous mitotic figures, focal necrosis, and formation of vascular spaces with focal intracellular vascular lumina and intracellular erythrocytes (Figure 2). Tumor cells were positive for CD31 and CD34, and were negative for Desmin, Keratin, and human herpesvirus 8 (HHV8) immunostains (Figure 3 and Figure 4). Mitotic rate was 21 per 10 high-power fields. Histologic Grade according to the French Federation of Cancer Centers Sarcoma Group (FNCLCC) was Grade 3 [1]. The mass appeared to be clinically aggressive intraoperatively with invasion of the atrial wall but was completely resected.
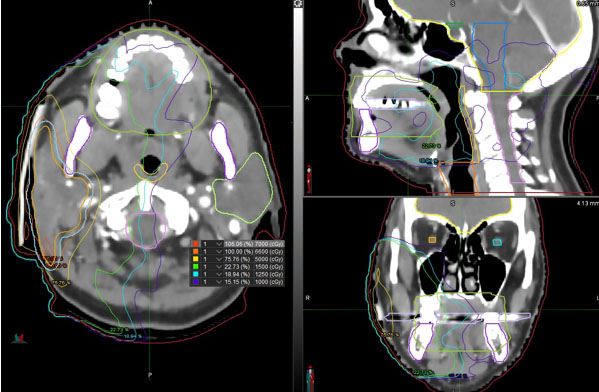
When subsequent imaging excluded distant disease, the patient was begun on adjuvant Adriamycin and Ifosfamide for stage pT2bNxMx cardiac angiosarcoma, histologic grade 3. His Eastern Cooperative Oncology Group performance status score was 0. He underwent four cycles of chemotherapy with Adriamycin/Ifosfamide without dose reduction. A repeat MRI following cycle four demonstrated a soft tissue lesion with non-specific signal characteristics adjacent to the atrial appendage. Given the aggressive nature of his disease, he was sent for external beam radiation therapy (EBRT) to both the site of his primary angiosarcoma in the lateral right atrium as well as a suspicious site in the right pericardium. He received a total tumor dose of 66 Gray in 33 daily fractions of 2 Gray each.
He was surveilled for four years until eventual relapse with biopsy-proven metastatic liver lesions consistent with known primary. Given his prior receipt of Adriamycin, he was begun on gemcitabine and docetaxel: Gemcitabine (900 mg/m2) on day 1, followed by Gemcitabine (900 mg/m2) with Docetaxel (80 mg/m2) on day 9. He was also started on a bisphosphonate for his bone metastases. Docetaxel was dose-reduced by 20% at initiation for chronic transaminitis and was dropped from his regimen following cycle six. He was thereafter maintained on gemcitabine monotherapy which he tolerated well, working full-time, and experienced over 36 months of clinical response on surveillance imaging, from March 2020 to June 2022.
Unfortunately, osseous progression was demonstrated on surveillance computed tomography (CT) in October 2022 and confirmed by subsequent bone scan. In the months leading to progression, the patient had been intermittently missing day 8 of multiple treatment cycles due to employment obligations. Adherence to therapy was reinforced and subsequent imaging in the following months demonstrated disease stability. To date, he continues to demonstrate durable response to gemcitabine monotherapy with improved treatment adherence.
Discussion
The differential for intracardiac tumor includes benign etiologies such as lipoma or other myxoma as well as malignant tumors such as sarcoma, lymphoma, or metastatic carcinoma; the diagnosis of cardiac angiosarcoma can only be achieved by acquisition of tissue. Most commonly occurring between the third and fifth decades of life, primary cardiac angiosarcomas are exceedingly rare and clinically aggressive. The majority are found in the right atrium and can spread locally or distantly with highly variable clinical manifestations based on the pattern of spread and tissue invasion.
Due to the difficulty of tumor resection, likelihood of advanced stage at diagnosis, and propensity for distant metastasis, prognosis of cardiac angiosarcoma is usually poor and is dependent on age at diagnosis, candidacy to resection, and stage of disease. Though data are scarce due to its clinical rarity, 5-year survival in cardiac angiosarcoma is estimated near 14% and a mean survival of only 3.8±2.5 months without surgical resection with median survival of just seven months and 5-year survival of 9.8% in all cardiac sarcoma [2],[3],[4],[5].
Surgical resection is a cornerstone of treatment not only for the sake of local control but for the preservation of cardiac function which influences patient morbidity and eligibility for cardiotoxic chemotherapy such as anthracyclines. For this reason, the survival benefit of resection has been shown to occur independently from the presence or absence of distant metastases. Irrespective of the completeness of resection, a multidisciplinary approach is still indicated for the prevention of relapse, although optimal treatment approach remains controversial [6].
In general, management of cardiac angiosarcoma has been guided by retrospective reviews of the literature and management of other noncardiac sarcoma. Doxorubicin-Ifosfamide (AI) and Gemcitabine-Docetaxel (GT) are often employed as first line treatment, although regimens including Pazopanib, Cisplatin, Cyclophosphamide, Dacarbazine, Mitomycin-C, Paclitaxel, and Vincristine have also been proposed [2],[7],[8],[9]. Finally, immunotherapy has emerged as a promising new avenue for achieving durable response in angiosarcoma. Although heretofore most available data are derived from retrospective reviews or single-institution studies, expanded clinical trials are underway [10].
Management of cardiac angiosarcoma remains a challenge for several reasons. Foremost is the diagnostic hurdle accompanying this rare malignancy, seldom suspected and often late-presenting due to non-specific and misleading symptomatology; in addition, it is difficult to obtain sufficient surgical histopathology outside of highly specialized centers. Further, due to its clinical rarity and aggressive nature, the available literature regarding therapeutic approach is restricted to smaller scale studies such as case series, retrospective reviews, and other forms of expert opinion [11],[12].
Our patient fits the expected demographics and tumor characteristics commonly seen in cardiac angiosarcoma. He was fortunate to receive complete tumor resection but ultimately did progress despite combination chemotherapy and EBRT. What is remarkable is his durable treatment response to single-agent gemcitabine and the close responsiveness of his disease with treatment adherence.
There are literature to support the use of single agent gemcitabine in this context. In a retrospective cohort study published by Watson et al. [13], 42 patients with pre-treated, advanced angiosarcoma of various subcategories including cardiac were assessed for clinical response and toxicity with single agent gemcitabine in the palliative setting. This study was concordant with prior literature in demonstrating clinical efficacy with a favorable side effect profile using single agent gemcitabine but analyzed only eight cases of cardiac angiosarcoma within the total cohort, highlighting the paucity of data guiding the use of this agent for this specific patient subpopulation [14]. Further work is needed to better understand the specific subpopulations for which this agent is an optimum choice.
Conclusion
This case adds to the existing literature suggesting the possibility of single-agent palliative chemotherapy in cardiac angiosarcoma for well-selected patients. As in this case, single-agent gemcitabine may be well tolerated over multiple cycles, preserving quality of life in the face of an otherwise aggressive malignancy.
REFERENCES
1.
Cates JMM. The AJCC 8th Edition staging system for soft tissue sarcoma of the extremities or trunk: A cohort study of the SEER database. J Natl Compr Canc Netw 2018;16(2):144–52. [CrossRef]
[Pubmed]

2.
Patel SD, Peterson A, Bartczak A, et al. Primary cardiac angiosarcoma – A review. Med Sci Monit 2014;20:103–9. [CrossRef]
[Pubmed]

3.
Siontis BL, Zhao L, Leja M, et al. Primary cardiac sarcoma: A rare, aggressive malignancy with a high propensity for brain metastases. Sarcoma 2019;2019:1960593. [CrossRef]
[Pubmed]

4.
Kumari N, Bhandari S, Ishfaq A, et al. Primary cardiac angiosarcoma: A review. Cureus 2023;15(7):e41947. [CrossRef]
[Pubmed]

5.
Yin K, Luo R, Wei Y, et al. Survival outcomes in patients with primary cardiac sarcoma in the United States. J Thorac Cardiovasc Surg 2021;162(1):107–15.e2. [CrossRef]
[Pubmed]

6.
Hamidi M, Moody JS, Weigel TL, Kozak KR. Primary cardiac sarcoma. Ann Thorac Surg 2010;90(1):176–81. [CrossRef]
[Pubmed]

7.
Suderman D, Cooke A, Wong R, Klein J. Treatment of cardiac angiosarcoma with radiation and docetaxel: A case report with partial response and prolonged stable disease. J Thorac Oncol 2011;6(4):834–5. [CrossRef]
[Pubmed]

8.
Inagaki C, Shimoi T, Okuma H, et al. A case of heavily pretreated metastatic cardiac angiosarcoma treated successfully using eribulin. Anticancer Drugs 2018;29(1):97–101.
[Pubmed]

9.
Honda K, Ando M, Sugiyama K, et al. Successful treatment of cardiac angiosarcoma associated with disseminated intravascular coagulation with Nab-Paclitaxel: A case report and review of the literature. Case Rep Oncol 2017;10(3):863–70. [CrossRef]
[Pubmed]

10.
Zeng Z, Mei Z, Chen M, et al. Cadonilimab plus anlotinib effectively relieve rare cardiac angiosarcoma with multiple metastases: A case report and literature review. Clin Res Cardiol 2024;113(2):358–65. [CrossRef]
[Pubmed]

11.
Shapiro LM. Cardiac tumours: Diagnosis and management. Heart 2001;85(2):218–22. [CrossRef]
[Pubmed]

12.
Krishnan T, Pettersson G, Mukherjee R, Singhal N. Cardiac angiosarcoma: A diagnostic and therapeutic challenge. J Cardiol Cases 2020;22(2):90–3. [CrossRef]
[Pubmed]

13.
Watson S, Verret B, Ropert S, et al. Single-agent gemcitabine in patients with advanced, pre-treated angiosarcoma: A multicenter, retrospective study. Cancer Med 2023;12(3):3160–6. [CrossRef]
[Pubmed]

14.
Stacchiotti S, Palassini E, Sanfilippo R, et al. Gemcitabine in advanced angiosarcoma: A retrospective case series analysis from the Italian Rare Cancer Network. Ann Oncol 2012;23(2):501–8. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Matthew Meranda - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gamze Gokturk-Ozcan - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Marian Girgis - Conception of the work, Design of the work, Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Matthew Meranda et al.. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.