|
Case Report
Nivolumab induced tenosynovitis which resolved without the use of steroids
1 Fellow, Hematology/Oncology, Brooke Army Medical Center, Fort Sam Houston, Texas, USA
2 Attending Physician, Hematology/Oncology, Brooke Army Medical Center, Fort Sam Houston, Texas, USA
Address correspondence to:
Eugen A Shippey
1118 Nolan Street, San Antonio, TX 78202,
USA
Message to Corresponding Author
Article ID: 100090Z10ES2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Shippey E, Setlik R. Nivolumab induced tenosynovitis which resolved without the use of steroids. J Case Rep Images Oncology 2021;7:100090Z10ES2021.ABSTRACT
Introduction: The advent of immunotherapy with PDL1 inhibitors like nivolumab has dramatically altered the oncology treatment armamentarium for advanced stage cancer and offers the possibility of long-term survival for some patients. However, these agents are associated with autoimmune adverse effects, some of which are still being characterized.
Case Report: Our case demonstrates reversible autoimmune tenosynovitis attributable to nivolumab. There have been four prior cases of autoimmune tenosynovitis associated with nivolumab use described in the literature. This is the first case described in which symptoms resolved rapidly without the use of steroids. Treatment was successfully restarted and tolerated for an additional year without further significant side effects.
Conclusion: This case is important because it demonstrates that some patients developing immune checkpoint inhibitor (ICI)-induced autoimmune tenosynovitis may continue cancer therapy with an ICI without concurrent immunosuppression and toxicities of systemic steroids.
Keywords: Autoimmune tenosynovitis, Checkpoint inhibitors, Immune-related adverse events, Melanoma, Nivolumab
Introduction
PD-L1 inhibitors and other immune checkpoint inhibitor (ICI) immunotherapies have improved the survival and quality of life of patients with numerous types of cancer [1]. In 2014, the US Food and Drug Administration (FDA) approved nivolumab for treatment of etastatic melanoma. Nivolumab has achieved 1 year survival rates of up to 73% in patients with previously untreated metastatic melanoma [2]. These agents are frequently associated with autoimmune side effects, potentially leading to long-term complications. Up to 80% of patients experience various inflammatory-mediated and immune-related adverse events (irAEs) [3].
Given that the number of indications for these agents has grown dramatically in the past decade, knowledge and mitigation of their side effects are paramount. Autoimmune tenosynovitis is not typically seen as an irAE and has been reported in only a handful of cases. A review of the associated literature revealed four other cases of nivolumab associated tenosynovitis, all of which required treatment with steroids [4],[5],[6]. Below we describe the case of a patient with autoimmune tenosynovitis attributable to nivolumab. In this case, his symptoms improved rapidly after holding nivolumab without using steroids. Treatment was successfully restarted, and he tolerated nivolumab for an additional year without further side effects.
Case Report
Our patient is a 67-year-old male with a medical history of metastatic recurrent BRAF-wild type melanoma. The patient was initially diagnosed with a Clark’s level I melanoma on the left cheek seven years prior. He developed recurrence within a left intraparotid lymph node four years later and underwent excision, left neck dissection (which showed no further metastatic disease), and radiation therapy. The patient’s other medical problems include severe chronic obstructive pulmonary disease (COPD) treated with inhalers and 2–3 L of continuous oxygen, stage I adenocarcinoma of the lung which due to his poor pulmonary function was treated with radiation only and was stable at the initiation of ICI therapy, and a remote history of prostate cancer for which he underwent a prostatectomy. He also had hypertension, reflux, chronic low back pain, and seasonal allergies, which were controlled with diltiazem, omeprazole, gabapentin, and atorvastatin, respectively. His performance status was 1 on the Eastern Cooperative Group (ECOG) scale, limited by dyspnea from COPD and from chronic low back pain from osteoarthritis.
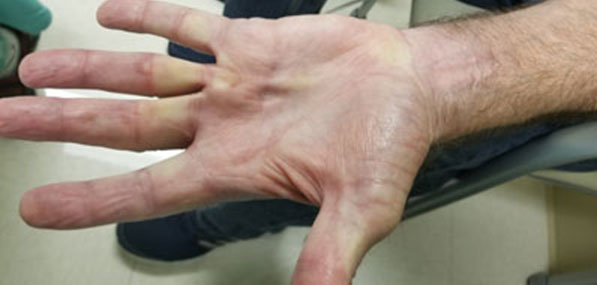
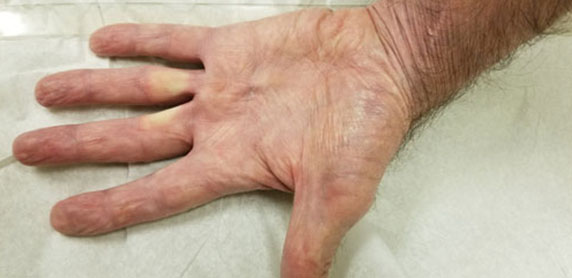
He remained without evidence of recurrent melanoma for the next three years, until routine surveillance positron emission tomography/computed tomography (PET/CT) demonstrated a new left lower lobe pulmonary nodule, the biopsy of which showed recurrent melanoma. He was started on nivolumab 240 mg every two weeks. During cycle 12 (his sixth month of treatment), he developed bilateral swollen flexor tendons to varying degrees of all fingers of his hands along with substantial accompanying tenderness and pain interfering with grasping objects, eating, and writing. He was able to perform activities of daily living (ADLs), such as feed and dress himself, though with significant discomfort. These symptoms rapidly developed over about one week. He could make a fist but could not relax it afterward due to severe pain in the palmar aspect of his hands. There was also erythema of the overlying skin (Figure 1). His fingers were otherwise not swollen, and he had no rheumatoid changes to the joints of his hands. Prior to onset of symptoms, he denied any trauma or medication changes. He had no medical history of auto-immune disorders, tendinopathies, osteoarthritis, soft tissue disorders, or chronic pain within his hands. He had no clinical evidence of infection. He had no other side effects other than chronic sequelae from his baseline COPD (for which he was not receiving oral or inhaled corticosteroids). His physical examination was normal. Considering the known association of nivolumab inducing soft tissue inflammation (and the patient having no similar past medical issues), nivolumab was held for two two-week cycles (about one month). During this period the swelling and pain in his hands greatly improved with slight residual pain in the flexor tendons and skin erythema (Figure 2), but he regained baseline function of his hands. Given the severity of his presenting symptoms, this irAE was considered Grade 3 of 5; i.e., affecting more than one finger or tendon; interfering with, but not preventing or limiting ADLs, and not requiring hospitalization or urgent intervention by Common Terminology for Adverse Events (CTCAE) Version 5.
Nivolumab was restarted successfully the following month and he received an additional year of treatment. He developed loose stools when nivolumab was switched from a two-week cycle to a four-week cycle. This symptom was not severe enough to require holding treatment and symptoms completely resolved after returning to the two-week treatment course. During further treatment he continued to have persistent mild soft tissue pain in the fingers with patchy overlying erythema (maximum CTCAE severity grade 1), but denied joint or bone pain and maintained normal baseline function of his hands.
Discussion
Above, we described a case of a patient with Grade 3 autoimmune tenosynovitis attributable to nivolumab which improved rapidly after holding nivolumab without using steroids. Treatment was successfully restarted and was tolerated for an additional year without further side effects. The patient’s tenosynovitis was not only temporally associated with therapy, but resolved when therapy was withdrawn, implicating nivolumab as the causative agent. No alternative etiology was identified as described above.
To our knowledge, there have been four other cases of nivolumab associated tenosynovitis reported in the literature [4],[5],[6]. Three of the four cases occurred during monotherapy; one was associated with PD-L1 and CTLA4 combination immunotherapy [5]. Mean time to appearance of joint involvement after initiation of immunotherapy was 4.4 months [4],[5],[6]. Three of the other cases were graded at 3 of 5 by CTCAE [7]. In one case, the patient became wheelchair bound and experienced significant limitations in ADLs [5]. Steroids were administered in all four cases to treat symptoms and were tapered over time as symptoms improved. In some cases, low to moderate doses of steroids were used to achieve a response [4],[5],[6]. In all cases, a response was achieved and in most cases nivolumab was restarted during a steroid taper. In one case, steroids were continued concurrently with immunotherapy long-term because of recurrent symptoms [5].
This case illustrates that symptoms of immunotherapy-induced soft tissue inflammation, which may significantly affect function and quality of life, may develop after prolonged treatment. In selected patients experiencing Grade 3 soft tissue inflammation as described above, these symptoms may abate quickly after holding treatment without use of systemic steroids. However, if such symptoms do not resolve or worsen (i.e., lead to worsening pain or limitation or prevention of ADLs), treatment with systemic steroids or other immunosuppressive agents should be initiated. For patients who solely require an interruption of ICI therapy, once symptoms have abated, it is reasonable to consider restarting immunotherapy and patients may remain asymptomatic afterward for an indefinite period.
Conclusion
As immunotherapy with ICIs becomes more widespread and further replaces traditional chemotherapy in first-line treatment of metastatic cancer, vigilant monitoring for adverse effects impacting quality of life should be of great importance to the treating physician. These side effects do not always require the same therapeutic approach. Less aggressive approaches may be appropriate and effective in selected patients. This case demonstrates that alleviation of the rarely seen irAE of autoimmune tenosynovitis can be achieved by holding immunotherapy without the use of systemic steroids. Patients may then be successfully restarted on an ICI for an indefinite period without recurrent side effects or long-term sequelae of soft tissue inflammation affecting subsequent quality of life or baseline function. This case adds to the literature describing the varying autoimmune phenomena associated with ICIs.
REFERENCES
1.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348(6230):56–61. [CrossRef]
[Pubmed]

2.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372(4):320–30. [CrossRef]
[Pubmed]

3.
Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: A systematic review of case reports. PLoS One 2016;11(7):e0160221. [CrossRef]
[Pubmed]

4.
Smith MH, Bass AR. Arthritis after cancer immunotherapy: Symptoms duration and treatment response. Arthritis Care Res (Hoboken) 2019;71(3):362–66. [CrossRef]
[Pubmed]

5.
Ngo L, Miller E, Valen P, Gertner E. Nivolumab induced remitting seronegative symmetrical synovitis with pitting edema in a patient with melanoma: A case report. J Med Case Rep 2018;12(1):48. [CrossRef]
[Pubmed]

6.
Gauci ML, Baroudjian B, Laly P, et al. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome induced by nivolumab. Semin Arthritis Rheum 2017;47(2):281–7. [CrossRef]
[Pubmed]

7.
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. November 27, 2017. [Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf]

SUPPORTING INFORMATION
Author Contributions
Eugen A Shippey - Conception of the work, Design of the work, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Robert Setlik - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Eugen Shippey et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.