|
Case Report
Basal cell carcinoma with extensive cerebellar invasion: A case report and literature review
1 Medical Doctor, Department of Neurosurgery, NTT Medical Center Tokyo, 5-9-22 Higashii-Gotanda, Shinagawa-ku,Tokyo, Japan
2 Medical Doctor, Department of Neurosurgery and Neuro-Oncology, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo, Japan
3 Chief Doctor, Department of Neurosurgery, Center Hospital of the National Center for Global Health and Medicine, 1-21-1 Toyama, Shinjuku-ku, Tokyo, Japan
4 Vice President, Department of Neurosurgery, Center Hospital of the National Center for Global Health and Medicine, 1-21-1 Toyama, Shinjuku-ku, Tokyo, Japan
Address correspondence to:
Ryuichi Noda
5-9-22 Higashii-Gotanda, Shinagawa-ku, Tokyo 141-8625,
Japan
Message to Corresponding Author
Article ID: 100076Z10RN2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Noda R, Yanagisawa S, Inoue M, Hara T. Basal cell carcinoma with extensive cerebellar invasion: A case report and literature review. J Case Rep Images Oncology 2021;7:100076Z10RN2021.ABSTRACT
Introduction: Basal cell carcinoma (BCC) is the most common malignant skin neoplasm with an increasing incidence worldwide. Its local destructive behavior can cause invasion to the deeper construction and reach the brain parenchyma.
Case Report: A 62-year-old male presented with myiasis of the occipital part of the skull with a large skin defect. Brain images showed a mass lesion in the cerebellum. The histological diagnosis, by biopsy of the skin lesion, was BCC.
Conclusion: Our case was an intradural infiltration of BCC, which was difficult to diagnose on initial presentation because the lesion was covered by myiasis. It is notable that neurosurgeons and oncologists should be aware of the natural history of BCC, since it could infiltrate into the brain, mimicking a case of intracranial tumor.
Keywords: Basal cell carcinoma, Intracranial infiltration, Metastasis, Myiasis
Introduction
Basal cell carcinoma is a non-melanocytic skin epithelial tumor arising from the basal layer cells of the epidermis and is the most common malignant skin neoplasm with an increasing incidence worldwide [1]. Seventy percent of BCC occurs in the head and neck [2], where sun exposure is the highest. Exposure to ultraviolet radiation is considered to be the main cause of BCC development [3]. It is characterized by local infiltration and destruction of surrounding tissue, but does not demonstrate aggressive invasion of deeper structures or significant metastatic tendencies [1],[4]. Therefore the risk of involvement of the skull and dura mater is very rare, and furthermore, intradural invasion is extremely rare [5]. Additionally, metastatic basal cell carcinoma (MBCC) is rarely seen. Because of these clinical features, the mortality rate of BCC is low [6]. However, years of neglect by the patient may lead to local invasion [7], metastasis and even death [8]. We report a case of BCC in an advanced stage of its natural history.
Case Report
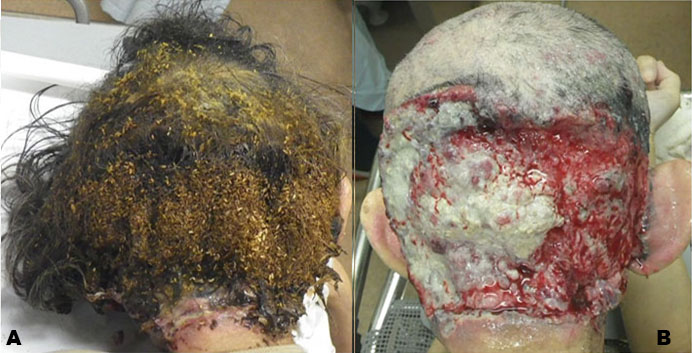
The patient was a 62-year-old male with a family history of schizophrenia. He realized a mass (7 mm in diameter) on his occipital scalp 22 years before his day of presentation. It had gradually grown for the next 10 years. Eleven years prior, it became a gray colored cauliflower-shaped mass, with some pus discharge. Since it caused no pain, the patient never sought medical treatment. The patient stated tearing off the mass several times in every few months, but had realized mass re-growth in the same place. Nine years prior, the mass began to grow bigger, and the skin started to deteriorate around the same time. One month before his presentation, he was unable to wake up and gradually became bedridden and malnourished. On the day of presentation, the patient told that the occipital part of his scalp had a large skin erosion covered with a swarm of maggots (Figure 1A). He presented to our emergency department with a friend. He had emaciation caused by long-term malnutrition. On physical examination, the patient was alert and conscious, and neurological examination revealed dysarthria and ataxia. After removal of the maggots, a large skin defect of the occipital scalp with bone exposure was revealed (Figure 1B). The skin defect was seen on the half of the occipital area of the scalp, covered with granulated tissue. Although there was no pus within the skin lesion or odor that suggested infection, visually it seemed to be a lesion of soft tissue infection due to the pressure sore caused by bedriddenness.
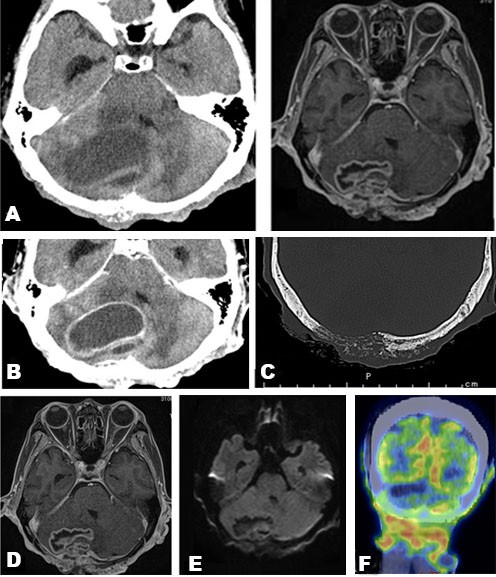
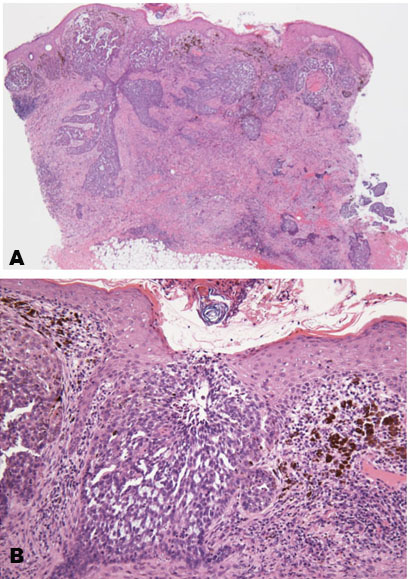
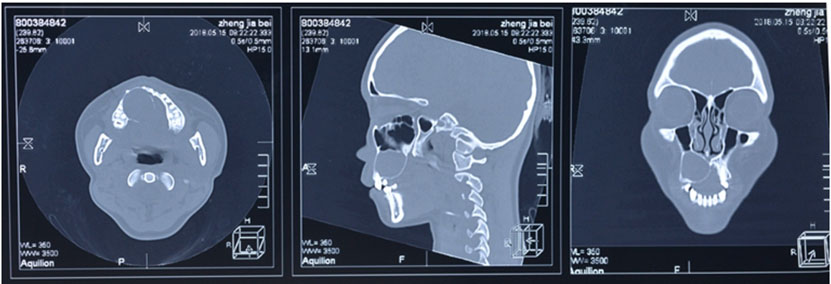
Blood examination showed elevated C-reactive protein levels. Computed tomography (CT) revealed a mass in the right cerebellum, obstructing the fourth ventricle and causing acute hydrocephalus (Figure 2A). On the bone window, the occipital bone had osteolytic change beneath the skin lesion (Figure 2C). The lesion had an enhanced cystic appearance on the contrast CT (Figure 2B). We suspected of brain abscess of the cerebellum caused by soft tissue infection. Emergency cyst drainage was performed. The aspirated fluid was serous and the microbiological examination was negative. Gadolinium-enhanced magnetic resonance imaging (MRI) was performed after the operation. Magnetic resonance imaging results showed shrinkage of the cyst, and the decreased mass effect improved the occlusive hydrocephalus (Figure 2D). There were no findings that indicated brain abscess on diffusion-weighted image (DWI) (Figure 2E). We performed an 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) CT scan to examine potential differential diagnoses, such as metastatic brain tumor or primary brain tumor. There was no uptake of FDG in the body or the cyst, suggesting an absence of primary cancer of the body. Instead, there was uptake on the occipital, nuchal skin (Figure 2F), and on the right side of the 12th thoracic vertebrae. Based on these findings, we suspected a skin tumor, and consulted dermatology. A punch biopsy of the occipital scalp was performed. Histologically, proliferation of tumor cells similar to basal cells is typically observed from the epidermis to the dermis. In the basal layer of the tumor, there was a gap between the palisading arrangement and surrounding connective tissue, mixed with melanocytes. Diverse and solid, lace-like proliferative images, and keratinized vesicles were also observed (Figure 3A and Figure 3B). Based on the histological findings, the diagnosis was BCC.
We also had a psychiatric consultation, given his family history of schizophrenia and his strange behavior observed during his hospitalization, such as incoherent speaking. The psychiatric diagnosis was schizophrenia, as expected. The patient refused further treatment, including surgery, chemotherapy, and radiation therapy. As such, the patient received best supportive care and subsequently passed away of hydrocephalus caused by tumor regrowth on the 44th day his hospitalization.
Discussion
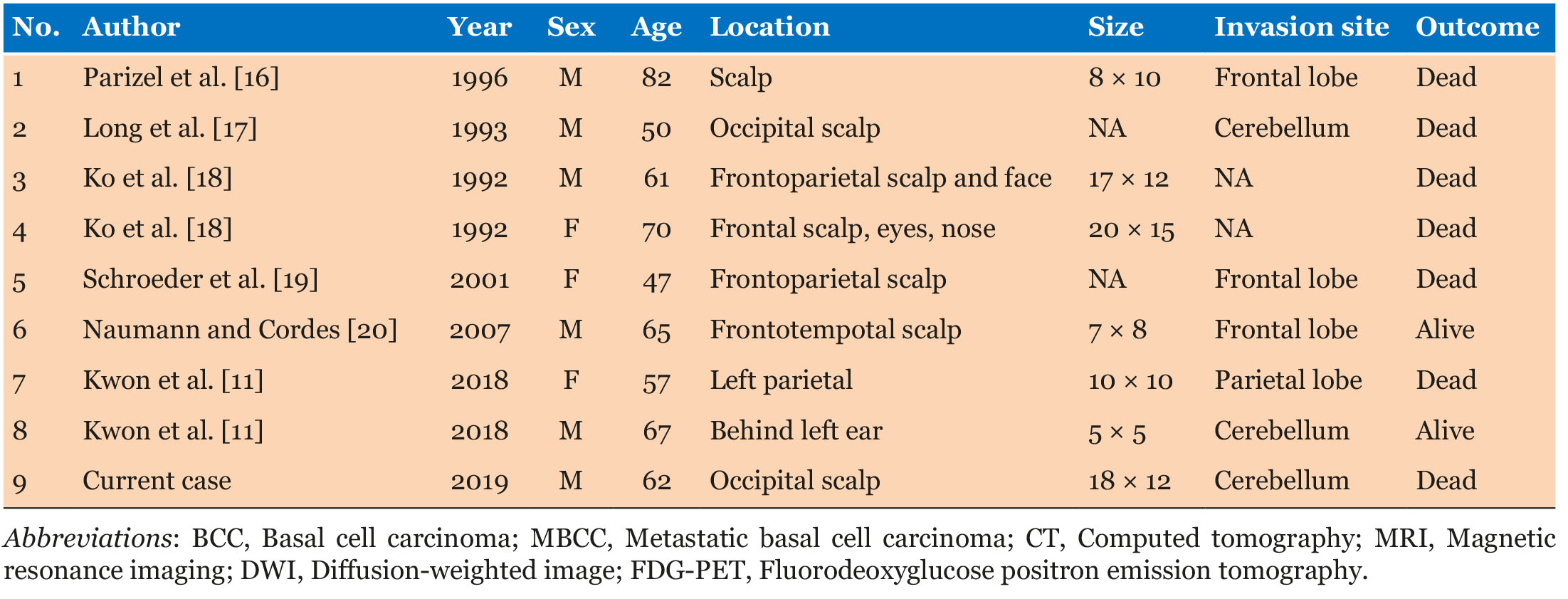
Basal cell carcinoma is the most common type of skin cancer, and is most commonly observed in older Caucasians (i.e., over 40 years of age) and on the face and necks. It tends to follow a benign clinical course characterized by slow local proliferation and very infrequent invasion of muscle, cartilage, or bone [7]. Because of its visibility, most are surgically removed within early stages of the disease, as a small skin lesion. For these reasons, distant metastasis and intracranial infiltration of BCC are very rare conditions in its natural progression. However, years of neglect by the patients or denial of medical attention allows the tumors to grow slowly over several years, leading to invasion and metastases of the disease. For example, this case was characterized by late patient presentation under family pressure [9]. Occasionally, a BCC may be lethal due to local tissue destruction if left untreated for a long period of time [8]. Our patient was brought to the emergency department after an emergency call made by a friend, after a long period of disregard for his condition. In cases of highly progressive local invasion, the tumor can infiltrate the subcutaneous tissue and dura mater, and reach the underlying brain parenchyma [10]. According to a PubMed search, 29 cases of intracranial invasion have been reported [7],[11],[12],[13],[14],[15]. There are only 8 cases of BCC with intradural tumor invasion in the literature [16],[17],[18],[19],[20], with our case being the 9th (see Table 1). Similar to the previous cases, our patient was an individual over the age of 50, and all cases had a distinct large skin lesion. There were only two cases of cerebellar invasion. Most of the patients in the identified articles had deceased. Accordingly, intradural infiltration of BCC is fatal.
Metastatic basal cell carcinoma is a rare condition. Depending on the type, the reported incidence of remote metastasis ranges from 0.0028% to 0.55% [21]. Previous articles reported that the primary tumor of MBCC is often large (>10 cm2) [21],[22],[23],[24],[25]. The size of the tumor predicts the tendency of metastasis. For instance, 1.9% of tumors >3 cm in diameter were metastatic, 50% if >10 cm diameter, and 100% if >25 cm diameter [22],[25],[26]. Our patient’s skin lesion was 18 cm in diameter, and it was clear and metastatic.
Myiasis is a disease caused by infestation of Dipteran larvae in human tissues, feeding on the tissue and body fluids [27]. It occurs more frequently in tropical countries but has been described in all regions [28],[29]. Previous articles have reported the occurrence of myiasis in skin cancer. The ulcerated, necrotic lesions exposed to the environment without wound dressings can cause the onset of myiasis in skin cancer. Risk factors for myiasis include older age, low socioeconomic status, poor hygiene, alcohol abuse, and individuals with chronic diseases such as diabetes mellitus or peripheral vascular disease that may cause chronic ulcers, psychiatric disorders, or lower conscience levels [30]. In fact, 30% of giant BCC cases are related to neglect. It was surprising that our patient kept neglecting the skin erosion with maggots. The reason for our patient’s neglect was likely an effect of his psychiatric disorder. The existence of myiasis clouded our judgment in making the right preoperative diagnosis.
Conclusion
In conclusion, our case was an intradural infiltration BCC with metastasis, which was difficult to diagnose on initial presentation because the lesion was covered by myiasis. It is notable that neurosurgeons and oncologists should be aware of the natural history of BCC, since it could infiltrate into the brain, mimicking a case of intracranial tumor.
REFERENCES
1.
Wong CSM, Strange RC, Lear JT. Basal cell carcinoma. BMJ 2003;327(7418):794–8. [CrossRef]
[Pubmed]

2.
von Domarus H, Stevens PJ. Metastatic basal cell carcinoma: Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol 1984;10(6):1043–60. [CrossRef]
[Pubmed]

3.
Situm M, Buljan M, Bulat V, Lugovic? Mihic? L, Bolanca Z, Simic? D. The role of UV radiation in the development of basal cell carcinoma. Coll Antropol 2008;32 Suppl 2:167–70.
[Pubmed]

4.
Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol 2002;146 Suppl 61:1–6. [CrossRef]
[Pubmed]

5.
Kleydman Y, Manolidis S, Ratner D. Basal cell carcinoma with intracranial invasion. J Am Acad Dermatol 2009;60(6):1045–9. [CrossRef]
[Pubmed]

6.
Cotran RS. Metastasizing basal cell carcinomas. Cancer 1961;14(5):1036–40. [CrossRef]
[Pubmed]

7.
Blackmon J, Machan M, Rajpara A, Beatty R. Recurrent basal cell carcinoma with intracranial invasion: A case report and literature review. Dermatol Online J 2014;20(7):13030/qt11t0g5h8.
[Pubmed]

8.
Kovarik CL, Stewart D, Barnard JJ. Lethal basal cell carcinoma secondary to cerebral invasion. J Am Acad Dermatol 2005;52(1):149–51. [CrossRef]
[Pubmed]

9.
Fattah A, Pollock J, Maheshwar A, Britto JA. Big Bad BCCs: Craniofacial resection and reconstruction for atypical basal cell carcinomata. J Plast Reconstr Aesthet Surg 2010;63(5):e433–41. [CrossRef]
[Pubmed]

10.
Donald PJ, Boggan J, Farwell DG, Enepekides DJ. Skull base surgery for the management of deeply invasive scalp cancer. Skull Base 2011;21(6):343–50. [CrossRef]
[Pubmed]

11.
Kwon CS, Awar OA, Ripa V, Said G, Rocka S. Basal cell carcinoma of the scalp with destruction and invasion into the calvarium and dura mater: Report of 7 cases and review of literature. J Clin NeuroSci 2018;47:190–7. [CrossRef]
[Pubmed]

12.
13.
Kondoff S, Drenchev A, Lotti T, et al. Successful craniotomy for advanced basal cell carcinomas with cranial bone invasion and dura mater infiltration – unique presentation in a Bulgarian patient. Open Access Maced J Med Sci 2018;6(2):372–5. [CrossRef]
[Pubmed]

14.
Mahvash M. Intracranial basal cell carcinoma with extensive invasion of the skull base. Turk Neurosurg 2014;24(4):571–3. [CrossRef]
[Pubmed]

15.
Maugeri R, Basile L, Giugno A, Graziano F, Iacopino DG. Impasse in the management of recurrent basal cell carcinoma of the skull with sagittal sinus erosion. Interdisciplinary Neurosurgery: Advanced Techniques and Case Management 2015;2(3):160–3. [CrossRef]

16.
Parizel PM, Dirix L, Van den Weyngaert D, et al. Deep cerebral invasion by basal cell carcinoma of the scalp. Neuroradiology 1996;38(6):575–7. [CrossRef]
[Pubmed]

17.
Long SD, Kuhn MJ, Wynstra JH. Intracranial extension of basal cell carcinoma of the scalp. Comput Med Imaging Graph 1993;17(6):469–71. [CrossRef]
[Pubmed]

18.
Ko CB, Walton S, Keczkes K. Extensive and fatal basal cell carcinoma: A report of three cases. Br J Dermatol 1992;127(2):164–7. [CrossRef]
[Pubmed]

19.
Schroeder M, Kestlmeier R, Schlegel J, Trappe AE. Extensive cerebral invasion of a basal cell carcinoma of the scalp. Eur J Surg Oncol 2001;27(5):510–1. [CrossRef]
[Pubmed]

20.
Naumann IC, Cordes SR. Giant basal cell carcinoma of the forehead with extensive intracranial involvement. Ann Otol Rhinol Laryngol 2007;116(9):663–6. [CrossRef]
[Pubmed]

21.
Ting PT, Kasper R, Arlette JP. Metastatic basal cell carcinoma: Report of two cases and literature review. J Cutan Med Surg 2005;9(1):10–15. [CrossRef]
[Pubmed]

22.
Snow SN, Sahl W, Lo JS, et al. Metastatic basal cell carcinoma: Report of 5 cases. Cancer 1994;73(2):328–35. [CrossRef]
[Pubmed]

23.
Oram Y, Orengo I, Alford E, Green LK, Rosen T, Netscher DT. Basal cell carcinoma of the scalp resulting in spine metastasis in a black patient. J Am Acad Dermatol 1994;31(5 Pt 2):916–20. [CrossRef]
[Pubmed]

24.
Farmer ER, Helwig EB. Metastatic basal cell carcinoma: A clinicopathologic study of seventeen cases. Cancer 1980;46(4):748–57. [CrossRef]
[Pubmed]

25.
Sahl WJ Jr, Snow SN, Levine NS. Giant basal cell carcinoma: Report of two cases and review of the literature. Am J Acad Dermatol 1994;30(5 Pt 2):856–9.
[Pubmed]

26.
Walling HW, Fosko SW, Geraminejad PA, Whitaker DC, Arpey CJ. Aggressive basal cell carcinoma: Presentation, pathogenesis, and management. Cancer Metastasis Rev 2004;23(3–4):389–402. [CrossRef]
[Pubmed]

27.
Ting PT, Barankin B. Cutaneous myiasis from Panama, South America: Case report and review. J Cutan Med Surg 2008;12(3):133–8. [CrossRef]
[Pubmed]

28.
Rubio C, Ladroón de Guevara C, Martiín MA, Campos L, Quesada A, Casado M. Cutaneous myiasis over tumor-lesions: Presentation of three cases. [Article in Spanish]. Actas Dermosifiliogr 2006;97(1):39–42. [CrossRef]
[Pubmed]

29.
Ibrahim SF, Pryor J, Merritt RW, Scott G, Beck LA. Cutaneous myiasis arising in an eccrine adnexal neoplasm. Dermatol Online J 2008;14(6):6.
[Pubmed]

30.
Raposo AA, Schettini APM, Massone C. Concurrent primary and secondary myiasis on basal cell carcinoma. An Bras Dermatol 2012;87(2):292–5. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Ryuichi Noda - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Shunsuke Yanagisawa - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Masato Inoue - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Tetsuo Hara - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Ryuichi Noda et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.