|
Case Report
Non-resolving skin rash with rising white cell count: An unusual cause
1 MD, Internal Medicine Resident, Department of Internal Medicine, Creighton University School of Medicine, Phoenix, Arizona, USA
2 MHA, Head of Hematology, Oncology Unit, Associate Professor, Department of Medicine, University of Arizona, Phoenix, Arizona, USA
Address correspondence to:
Arnold Nongmoh Forlemu
MD, 2601 E Roosevelt St, Phoenix, Arizona 85008,
USA
Message to Corresponding Author
Article ID: 100066Z10AF2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Forlemu AN, Kosa D, Amar S. Non-resolving skin rash with rising white cell count: An unusual cause. J Case Rep Images Oncology 2020;6:100066Z10AF2020.ABSTRACT
Introduction: Rash with leukocytosis is a common complaint in primary-care-clinics and the differential diagnosis is varied including infections, allergies, autoimmune disorders, and drug reactions. Malignancies are usually much lower in this differential diagnosis.
Case Report: We present a patient with T-cell prolymphocytic leukemia who went undiagnosed for more than a year.
Conclusion: This case highlights the fact that T-cell malignancies may be missed if the only presentation is a rash. Likewise, the case raises awareness to suspect such malignancies in patients with non-resolving rash and rising leukocytosis.
Keywords: Skin rash, T-cell prolymphocytic leukemia, Unresolving rash
Introduction
T-cell prolymphocytic leukemia (T-PLL) is a rare and very aggressive malignancy characterized by the clonal proliferation of lymphocytes [1]. It often presents with splenomegaly, lymphadenopathy, and cutaneous lesions, and has a very poor prognosis with traditional chemotherapy [1],[2].
When it manifests only as a rash, it may be confused with other more common dermatologic lesions. Anti-CD52 monoclonal antibody has considerably improved outcomes; however, an autologous or allogeneic stem cell transplantation which further prolongs survival may offer potential cure.
Case Report
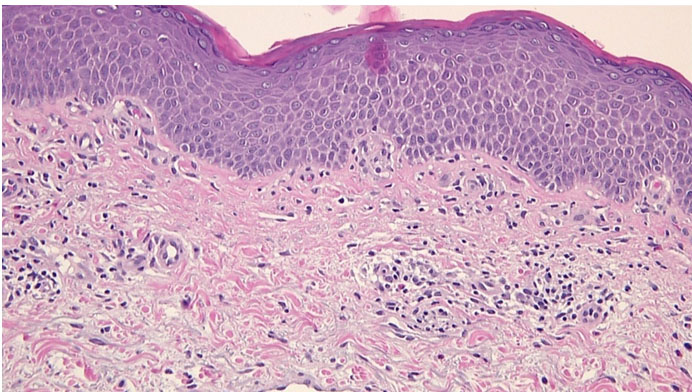
A 70-year-old man presented with fatigue for four months without B symptoms. He had a rash in both inguinal folds, 15 months prior to his presentation. At the time, he consulted a dermatologist and a skin biopsy was done which revealed a subacute spongiotic dermatitis rich in lymphocytes (Figure 1). He was treated with topical steroids/antifungals and oral antibiotics with minor improvement and subsequent relapse. At the time his white cell count (WBC) was 9600/uL. Patient reported having a WBC of 24,000/uL and 77,000/uL five months and three months ago, respectively.
At time of our evaluation he had bilateral groin pruritic erythematous papular/scaly plaques with inguinal lymphadenopathy measuring 2 × 2 cm right and 1 × 1 cm on left (Figure 2).
The patient’s complete blood count showed a WBC of 132,400/μL (86% lymphoid cells), a hemoglobin (Hb) of 10.8 g/dL, and a platelet count of 101,000/μL. A peripheral blood smear revealed 70–80% atypical lymphoid cells with nucleolysis and morphology consistent with prolymphocytes. A human T-cell leukemia virus 1 done was negative.
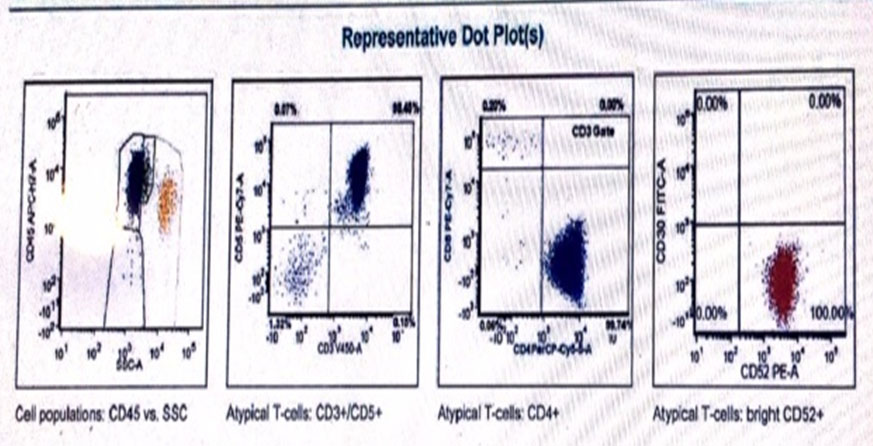
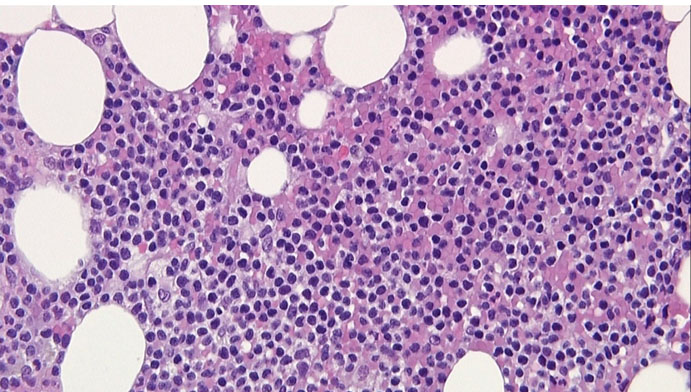
In view of the clinical picture of a non-resolving skin lesion with lymphocytosis, a diagnosis of possible T-cell malignancy was made. A flow cytometry showed 90% atypical T-cells with positive CD45, CD2, CD3, CD4, CD5, CD7, and CD52 (Figure 3). A bone marrow biopsy showed 50–80% cellularity with atypical lymphoreticular infiltrate (Figure 4), and immunochemistry was consistent with flow cytometry findings. Immunoperoxidase stain for CD16, CD25, CD30, CD56, CD117, CD1a, and TdT were negative, and clonal T-cell receptor (TCR) beta gene rearrangement tests were positive. The bone marrow biopsy also showed an abnormal male karyotype 47XY, +8 [2]/46,XY [3] suggestive of Trisomy 8. A computed tomography (CT) scan revealed splenomegaly (15 cm) and diffuse lymphadenopathy (supraclavicular, hilar, axillary, inguinal, mediastinal). The patient was diagnosed with T-PLL and transferred to a leukemia center to receive treatment.
Discussion
T-cell prolymphocytic leukemia is a rare but aggressive mature T-cell malignancy that accounts for less than 2% of mature lymphocytic leukemias and has a poor prognosis [2]. It is usually encountered in adults >65 years or older, with a 2:1 male to female ratio [3]. T-cell prolymphocytic leukemia can present with fatigue, splenomegaly (>80%), lymphadenopathy (50%), skin lesions (<30%), leukocytosis (70%) [2],[4],[5]. Skin lesions can include skin nodules, maculopapular rash, or erythroderma and can predate the leukocytosis [6]. Like in our case, T-PLL may be misdiagnosed as eczema or fungi especially if this is the only presenting symptom. Patients often have a white cell count >100,000/μL and a hemoglobin of 10 g/dL at presentation [2]. Peripheral smear generally shows a classic prolymphocytic morphology with smallto-medium-sized cells, high nuclear/cytoplasmic ratio, a prominent nucleolus with a condensed chromatin, and basophilic cytoplasm with blebs. This may differentiate T-PLL from T-cell large granular lymphocytic leukemia which have usually large lymphocytes with azurophilic granules [1]. T-cell prolymphocytic leukemia cell nuclei may be regular round or oval, or they may be irregular and convoluted. However, in a few cases, the cells may be small or cerebriform and indistinguishable from chronic lymphocytic leukemia (CLL) cells, and the nucleolus may not be visible under light microscopy, hidden by the dense chromatin. Flow cytometry and immunohistochemistry are diagnostic (T-cell markers +) with characteristic clonal TCR gene rearrangement [1],[7]. T-cell prolymphocytic leukemia is often TdT-, CD1a-, CD2+, CD5+, CD7+, CD16-, CD56-, with variable CD4 and CD8 expression. In all cases of T-PLL, TCR b and/or g chain genes are rearranged. Most T-PLL cases express high densities of CD52, which is a glycosylphosphatidylinositol-linked protein present on both normal and malignant lymphocytes and is a frequent therapeutic target for T-PLL [1],[2],[3]. CD7 positivity differentiates T-PLL from other mature T-cell leukemias. Likewise, T-PLL and B-PLL are differentiated by the presence of lymphadenopathy, skin involvement, and immune histochemistry in T-PLL [1]. Bone marrow biopsy shows prolymphocyte infiltrates with a mixed diffuse and interstitial pattern and reticulin fibrosis. Skin biopsy may show perivascular, periadnexal, or diffuse dermal infiltrates with irregular medium-sized lymphocytes without epidermotropism [1]. Tissue biopsy, such as splenic biopsy, demonstrating red pulp infiltration may be necessary in patients with unusual presentation [3]. Similar to our case, T-PLL may be associated with chromosomal aberrations mainly chromosomes 14, 8, 11, and X [8], which may explain its aggressiveness. The prognosis of T-PLL is poor with a median survival rate of seven months with conventional chemotherapy [1],[3]. Anti-CD52 monoclonal antibody is the first line of treatment (improves survival from 6 to18 months). Patients often need allogeneic stem cell transplantation, and this can extend survival to four years [1].
Conclusion
This case illustrates the potential to miss the diagnosis of a rare but highly aggressive hematologic malignancy that may present only as a skin rash. Lack of specific clonal T-cell testing on skin biopsy can miss the diagnosis. Rising WBC with lymphadenopathy and non-resolving rash should raise suspicion for T-cell malignancies.
REFERENCES
1.
Laribi K, Lemaire P, Sandrini J, Baugier de Materre A. Advances in the understanding and management of T-cell prolymphocytic leukemia. Oncotarget 2017;8(61):104664–86. [CrossRef]
[Pubmed]

2.
Graham RL, Cooper B, Krause JR. T-cell prolymphocytic leukemia. Proc (Bayl Univ Med Cent) 2013;26(1):19–21. [CrossRef]
[Pubmed]

3.
Vivekanandarajah A, Atallah JP, Gupta S. T-cell prolymphocytic leukaemia (T-PLL): A rare disease with a grave prognosis. BMJ Case Rep 2013;2013. pii: bcr2013009808. [CrossRef]
[Pubmed]

4.
Jeong KH, Lew BL, Sim WY. Generalized leukaemia cutis from a small cell variant of T-cell prolymphocytic leukaemia presenting with exfoliative dermatitis. Acta Derm Venereol 2009;89(5):509–12. [CrossRef]
[Pubmed]

5.
Robak T, Robak P. Current treatment options in prolymphocytic leukemia. Med Sci Monit 2007;13(4):RA69–80.
[Pubmed]

6.
Ashturkar AV, Pathak GS, More YE, Bhandare A. T-cell prolymphocytic leukemia with cutaneous involvement as the presenting feature. Indian J Dermatol 2012;57(2):157–8. [CrossRef]
[Pubmed]

7.
Serra A, Estrach MT, Marti R, Villamor N, Rafel M, Montserrat E. Cutaneous involvement as the first manifestation in a case of T-cell prolymphocytic leukaemia. Acta Derm Venereol 1998;78(3):198–200. [CrossRef]
[Pubmed]

8.
Tirado CA, Starshak P, Delgado P, Rao N. T-cell prolymphocytic leukemia (T-PLL), a heterogeneous disease exemplified by two cases and the important role of cytogenetics: A multidisciplinary approach. Exp Hematol Oncol 2012;1(1):21. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Arnold Nongmoh Forlemu - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dimas Kosa - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Surabhi Amar - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2020 Arnold Nongmoh Forlemu et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.