|
Case Report
Human epidermal growth factor 2 (HER2) non-small cell lung cancer (NSCLC) with YVMA mutation responsive to ado-trastuzumab emtansine
1 Brooke Army Medical Center, San Antonio, Texas, USA
Address correspondence to:
Kristin Stoll
DO, San Antonio Uniformed Health Consortium, San Antonio Military Medical Center, 500 Roger Brooke Drive, San Antonio, Texas 78234,
USA
Message to Corresponding Author
Article ID: 100065Z10KS2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Stoll K, Peterson M. Human epidermal growth factor 2 (HER2) non-small cell lung cancer (NSCLC) with YVMA mutation responsive to ado-trastuzumab emtansine. J Case Rep Images Oncology 2020;6:100065Z10KS2020.ABSTRACT
Human epidermal growth factor 2/NEU (HER2) aberrations account for 4.7–10% of all non-small cell lung cancers (NSCLC), making it one of the more prevalent forms of NSCLC. Human epidermal growth factor 2 aberrations also instigate breast cancer and these patients commonly demonstrate a robust response to HER targeted immunotherapies. This optimistically promised that HER2 NSCLC might also respond via the same pathophysiologic mechanisms. To date, this promise has largely failed to deliver, and an early trial ended in cancellation due to lack of efficacy. Contrary to this lack of efficacy, this case presents a patient with HER2 positive NSCLC with a specific gene amplification that demonstrated response to HER2 therapy. This adds to the growing evidence that targeted therapy can be efficacious in some HER2 positive NSCLC. It further reinforces the need for extended mutational testing in lung cancer patients, particularly those with a smoking history.
Keywords: Adenocarcinoma of the lung, HER2, HER2 positive lung cancer, NSCLC, Trastuzumab emtansine
Introduction
Human epidermal growth factor 2/NEU (HER2) aberrations account for 4.7–10% of all non-small cell lung cancers (NSCLC) [1], making it one of the more prevalent forms of NSCLC. It is most ubiquitous among nonsmoking women in middle age [2]. The HER2 receptor is a membrane-bound member of the tyrosine kinase family. It interacts with a diverse array of ligands and is found in a variety of tissues [3]. Human epidermal growth factor 2 aberrations also occur in approximately 15–25% of breast cancers and have been largely responsive to HER2 targeted therapies, including ado-trastuzumab emtansine, an antibody drug conjugate against HER2 [4],[5]. The efficacy of targeted therapy in breast cancer gave way to the hypothesis that targeted therapies would also prove effective in treating HER2 mutations in NSCLC. This hypothesis has largely failed to come to fruition, as evidenced most recently by the early termination A Phase II Study of trastuzumab emtansine in HER2-positive non-small cell lung cancer due to limited efficacy [6],[7].
A small number of case reports document response to HER2-targeted therapies in a specific HER2 mutation with a 12 base pair insertion Yampa Valley Medical Associates (YVMA) on exon 20 [8],[9],[10],[11]. We present a patient with the YVMA amplification demonstrating response to HER2 therapy, adding to the accumulating evidence that targeted therapy can be efficacious in HER2 positive NSCLC.
Case Report
A 65-year-old lifetime nonsmoking female was referred to the hematology/oncology service following primary evaluation for six months of fatigue and cough that was not productive of sputum with associated chest wall pain. Her past medical history was significant for hypothyroidism, osteopenia, seasonal allergic rhinitis, acid reflux, and depression with a remote history of seizures two decades prior to presentation. At the time of presentation, she was taking levothyroxine, montelukast, fluticasone spray, azelastine, omeprazole, citalopram, bupropion, and levetiracetam. Lab results at the time of diagnosis were notable for mild hyponatremia with sodium of 132 mmol/L, creatinine of 1.09 mg/dL elevated above her baseline of 0.7–0.9 mg/dL, leukocytosis to 12.1 × 103 without anemia and an elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) of 12.9 mg/dL and 60 mm/hour, respectively.
Computed tomography (CT) of the chest revealed a large spiculated mass in the left lower lobe measuring 7.2 cm in dimension and concerning. A follow-up transbronchial biopsy revealed fragments of lung parenchyma with atypical infiltrative glands adjacent to a fragment of ciliated respiratory epithelium and a background of desmoplastic reaction. These glands demonstrated atypical nuclei with membrane irregularities, some prominent nucleoid and increased mitoses. Performance of immunohistochemistry showed malignant cells positive for CK7 and focally positive for CK20 and CDX2. The malignant cells were negative for TTF-1, napsin, p40, CK5/6, and Pax-5, reflective of adenocarcinoma. This data, however, initially confused the primary source. Based on the immunohistochemical profile, the source might have been a primary lung or an upper aerodigestive or pancreaticobiliary adenocarcinoma. Staging with proton emission tomography (PET) demonstrated an area of increased metabolic activity within the wall of the cecum and the ascending colon. Given the immunohistochemical profile along with the area of enhancement, an upper endoscopy and colonoscopy were performed. These studies returned without evidence of mass or nodules. After review, the clinical and radiographic picture was most consistent with primary lung adenocarcinoma with metastatic pulmonary nodules in the right upper and lower lobes along with additional nodules in the left lower lobe independent of the spiculated mass.
Platinum doublet therapy with carboplatin and paclitaxel was initiated. She underwent six complete 21 day cycles of paclitaxel 340 mg and carboplatin 1700 mg intravenously on day one. Therapy was ultimately terminated due to toxicities after cycle six. She was transitioned to maintenance therapy with pemetrexed 500 mg/m2 intravenously given on cycle day one, but ultimately demonstrated disease progression after two 21 day cycles.
After a short course of palliative radiation therapy, she transitioned to a course of nivolumab 200 mg intravenously on day one of a 21 day cycle. After two cycles of therapy with nivolumab, she presented to the clinic with a progressively worsening dry cough, similar to her initial presentation. Her CT chest again demonstrated disease progression. At this point, various treatment options were discussed with the patient, including clinical trials.
Genetic testing of her carcinoma was performed during her initial evaluation ultimately revealed a HER2/ERBB2 mutation at exon 20 with A775_G776insYVMA amplification, a well-established HER2 aberration. At the time of this discussion, a phase II basket trial in patients with HER2 aberrations treated with ado-trastuzumab emtansine (T-DM1) had demonstrated a 30% response rate with a few long-term responders. A collaborative decision was made to start the patient on ado-trastuzumab emtansine, a HER2-antibody drug conjugate with selective incorporation into HER2 overexpressing cells resulting in apoptosis. Prior to the start of this therapy, the left lower lung mass measured 3.8 × 7.7 cm on chest CT.
Treatment
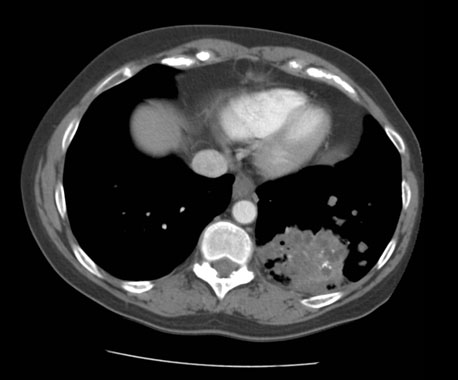
The patient started T-DM1 189 mg intravenously on day one of a 21 day cycle. A repeat chest CT two months after initiation of therapy demonstrated response with a decrease in the size of the left lower lung mass to 5.2 cm in diameter, representing a 32% reduction in size (Figure 1). This was also associated with a decrease in the size and number of additional pulmonary nodules. She tolerated the therapy February to August 2018 without disease progression. She experienced myelosuppression with severe thrombocytopenia evidenced by an undetectable white blood cell count and ultimately required a dose adjustment of the therapy.
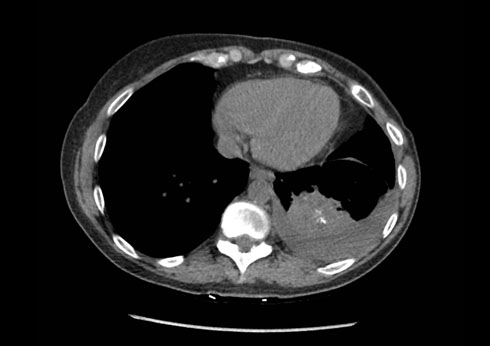
A subsequent chest CT at nine months following the initiation of therapy revisualized the left lung mass at 5.8 cm in diameter, representing an 11% increase in size (Figure 2). At that time, she was said to have had disease progression. She was started on immunotherapy with pembrolizumab and was further referred for a clinical trial in December 2018.
Outcome and Follow-Up
The patient was enrolled in a clinical trial with poziotinib an irreversible pan-HER tyrosine kinase inhibitor with efficacy against estimated glomerular filtration rate (EGFR), HER2, and HER4. She presented to the clinic for follow-up 21 months after her initial diagnosis and continues to do well in her clinical trial with stable disease.
Discussion
The case presented here adds to the accrued literature that demonstrated response to HER2 targeted therapy in patients with the A775_G776insYVMA aberration. The majority of mutations in the HER2 kinase domain in NSCLC have been identified as in-frame duplications or insertions in a small 8-codon region of exon 20 leading to an anomalous YVMA protein expression. This alteration causes a shift that narrows the ATP binding site, driving both an increase in tyrosine kinase activity and inhibitor sensitivity [12],[13]. It is a well-established oncogene driver in adenocarcinoma of the lung. A775_G776insYVMA amplification is the most common known mutation in HER2 NSCLC, representing approximately 60% of the pathology [14],[15].
Trastuzumab emtansine (T-DM1), the agent utilized in this case report, is an antibody-drug conjugate that enables intracellular drug delivery to HER2 overexpressing cells by utilizing the HER2-targered antitumor therapy of trastuzumab with the cytotoxic properties of the maytansinoid microtubule-inhibitory agent emtansine [16],[17]. Several phase III clinical trials for this therapy have repeatedly established efficacy in the treatment of advanced or relapsed HER2 positive breast cancer [18].
Extrapolated from the research in oncogene targeted therapy in HER2 positive breast cancer, studies on the Calu 3 lung carcinoma cell line demonstrated preclinical dose-dependent inhibition of cell growth after treatment with T-DM1 [13]. Further, murine studies of lung adenocarcinoma expressing HER2 insertion mutations on exon 20 demonstrated tumor regression when expression was inhibited with trastuzumab. This ultimately suggested that an exon 20 YVMA mutation could act as a potential therapeutic target in adenocarcinoma of the lung [15]. A lack of prospective clinical trials to address this topic prompted the phase II clinical trial in Japan specifically to evaluate T-DM1 in patients with HER2 expressing adenocarcinoma of the lung. This trial was ultimately terminated due to failure to achieve the primary endpoint of objective response rate, based on Response Evaluation Criteria in Solid Tumors.
This trial had a few notable limitations. It utilized the immunohistochemistry scoring system for HER2 positivity utilized for gastric cancer, rather than the stricter immunohistochemistry classification utilized for breast cancer. This more relaxed scoring system could have overrepresented the HER2 positivity of the study. It was further limited by its small size. Finally, it incorporated all known insertion mutations and did not breakdown results based on the specific aberration. This is important to comment upon the given number of case reports, including this one, that document response to HER2 therapy in patients with the A775_G776insYVMA amplification.
This perhaps suggests that the A775_G776insYVMA mutation still reflects a potential therapeutic target, requiring additional study. Mutation specific genetics should be considered in the design of further clinical trials of HER2 oncogene specific therapy in this aggressive and difficult to treat pathology. Given the clinical response of this patient, nonsmoking patients with lung cancer should be referred for genetic testing to determine potential responsiveness to oncogene therapy. Indeed, a case could be made to consider genetic testing in all patients given the results. Additionally, this adds to the growing body of literature supporting oncogene-based precision therapy for NSCLC.
Conclusion
Despite the success heralded by HER2 positive breast cancer, HER2 positive NSCLC has been largely nonresponsive to HER2 targeted therapy and has resulted in the early termination of at least one clinical trial due to lack of efficacy.
- Notwithstanding, there is growing evidence that specific mutations such as the A775_G776insYVMA amplification are favorably responsive to HER2 targeted therapy.
- Consequently, extended mutational testing in lung cancer is essential in nonsmokers and should be considered in all patients.
REFERENCES
1.
Kuyama S, Hotta K, Tabata M, et al. Impact of HER2 gene and protein status on the treatment outcome of cisplatin-based chemoradiotherapy for locally advanced non-small cell lung cancer. J Thorac Oncol 2008;3(5):477–82. [CrossRef]
[Pubmed]

2.
Shan L, Qiu T, Ling Y, et al. Prevalence and clinicopathological characteristics of HER2 and BRAF mutation in Chinese patients with lung adenocarcinoma. PLoS One 2015;10(6):e0130447. [CrossRef]
[Pubmed]

3.
Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol 2001;12 Suppl 1:S3–8. [CrossRef]
[Pubmed]

4.
King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 1985;229(4717):974–6. [CrossRef]
[Pubmed]

5.
Peddi PF, Hurvitz SA. Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: Latest evidence and clinical potential. Ther Adv Med Oncol 2014;6(5):202–9. [CrossRef]
[Pubmed]

6.
Harada D, Kozuki T, Nogami N, et al. MA 07.11 a phase II Study of trastuzumab emtansine in HER2- positive non-small-cell-lung cancer. Journal of Thoracic Oncology 2017;12(11 Suppl 2):S1829. [CrossRef]

7.
Wang Y, Zhang S, Wu F, et al. Outcomes of Pemetrexed-based chemotherapies in HER2-mutant lung cancers. BMC Cancer 2018;18(1):326. [CrossRef]
[Pubmed]

8.
Weiler D, Diebold J, Strobel K, Aebi S, Gautschi O. Rapid response to trastuzumab emtansine in a patient with HER2-driven lung cancer. J Thorac Oncol 2015;10(4):e16–7. [CrossRef]
[Pubmed]

9.
Shi Y, Wang M. Afatinib as first-line treatment for advanced lung adenocarcinoma patients harboring HER2 mutation: A case report and review of the literature. Thorac Cancer 2018;9(12):1788–94. [CrossRef]
[Pubmed]

10.
Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med 2006;354(24):2619–21. [CrossRef]
[Pubmed]

11.
Li BT, Lee A, O’Toole S, et al. HER2 insertion YVMA mutant lung cancer: Long natural history and response to afatinib. Lung Cancer 2015;90(3):617–9. [CrossRef]
[Pubmed]

12.
Koga T, Kobayashi Y, Tomizawa K, et al. Activity of a novel HER2 inhibitor, poziotinib, for HER2 exon 20 mutations in lung cancer and mechanism of acquired resistance: An in vitro study. Lung Cancer 2018;126:72–9. [CrossRef]
[Pubmed]

13.
Perera SA, Li D, Shimamura T, et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc Natl Acad Sci U S A 2009;106(2):474–9. [CrossRef]
[Pubmed]

14.
Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18(18):4910–8. [CrossRef]
[Pubmed]

15.
Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol 2015;26(7):1421–7. [CrossRef]
[Pubmed]

16.
Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008;68(22):9280–90. [CrossRef]
[Pubmed]

17.
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011;128(2):347–56. [CrossRef]
[Pubmed]

18.
Dhillon S. Trastuzumab emtansine: A review of its use in patients with HER2-positive advanced breast cancer previously treated with trastuzumab-based therapy. Drugs 2014;74(6):675–86. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Kristin Stoll - Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Matthew Peterson - Conception of the work, Design of the work, Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2020 Kristin Stoll et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.