|
Case Report
Temozolomide regulates primary central nerve system lymphoma, being resistant to high dose methotrexate-based chemotherapy and radiation
1 MD, Director, Department of Neurosurgery, Hokuto Hospital, Obihiro, Hokkaido, Japan
Address correspondence to:
Akira Tempaku
7-5, Inada-cho-kisen, Obihiro, Hokkaido 080-0833,
Japan
Message to Corresponding Author
Article ID: 100140Z10AT2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Tempaku A. Temozolomide regulates primary central nerve system lymphoma, being resistant to high dose methotrexate-based chemotherapy and radiation. J Case Rep Images Oncology 2025;11(1):1–4.ABSTRACT
Introduction: Primary central nerve system lymphoma (PCNSL) is an intracranial malignancy. Combined chemotherapy with methotrexate, procarbazine, vincristine, and Rituximab, following radiation, sometimes results in partial response or progressive disease. Temozolomide (TMZ) administration has been proposed to salvage therapy.
Case Report: A 72-year-old man had headache and forgetfulness. Mild disorientation was observed. Head magnetic resonance image revealed a mass lesion in the right frontal lobe. Pathological examination after an incisional biopsy revealed a diagnosis of diffuse large B cell lymphoma. Standard treatment consisted of 6 cycles of high-dose methotrexate-based chemotherapy with procarbazine and vincristine. Three cycles of high-dose methotrexate-based chemotherapy with procarbazine and vincristine, combined with Rituximab. Further, focal radiation therapy against core lesion with marginal area irradiation was performed. In spite of total standard treatment, the lesion was not under regulation. The patient continued TMZ therapy 29 cycles on an outpatient basis due to psychiatric complications that made it difficult to continue intravenous treatment in the hospital. The tumor mass and surrounding edematous lesions decreased.
Conclusion: Oral TMZ administration was effective in an elderly patient with PCNSL, who had failed standard chemotherapy and radiation therapy. It was less invasive, allowed for transition to outpatient management, and provided adequate therapeutic benefit. Future indications for similar cases should be considered.
Keywords: Methotrexate, Primary central nerve system lymphoma, Salvage therapy, Temozolomide
Introduction
Primary central nerve system lymphoma (PCNSL) is an intracranial malignancy that represents less than 5% of brain tumors [1], [2]. They tend to be more common in the elderly, spread diffusely, and have a poor prognosis. In recent years, high-dose methotrexate [3], [4], methotrexate with Rituximab [5], and radiation therapy [6], [7] have been shown to control the disease well. Combined chemotherapy with methotrexate, procarbazine, vincristine, and Rituximab also available and widely applied to PCNSL patients. However, some patients relapse even after combined chemotherapy and radiation therapy. The choice of additional therapy to them is often difficult. The efficacy of temozolomide (TMZ) as an additional chemotherapeutic agent has been suggested. Sufficient medical evidence has not been obtained [8], [9], [10].
This case report highlights a patient diagnosed with PCNSL who had inadequate tumor control after high-dose methotrexate-based chemotherapy (including Rituximab) and radiation therapy, but achieved tumor shrinkage with TMZ administration.
Case Report
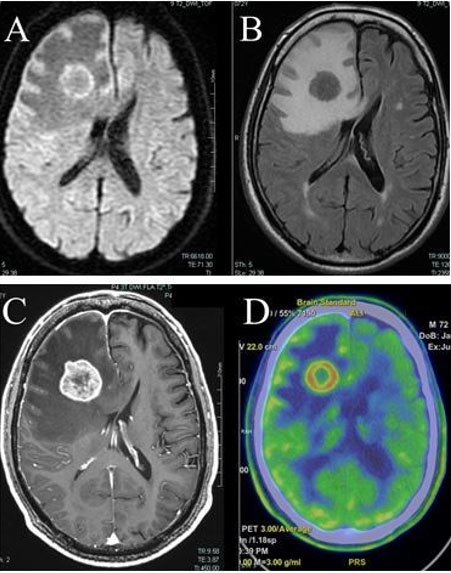
A 72-year-old man had headache and forgetfulness for the past two weeks. He had medical history of hypertension, dyslipidemia, and internal carotid artery stenosis (asymptomatic). No glucose intolerance noted. He had medications of calcium channel blocker, statin, and antiplatelet agent (clopidogrel 75 mg per day). He had 7 cigarettes to smoke, 3 glasses of whiskey, and 350 mL of beer to drink each day. Since slight cognitive dysfunction (21 points in Mini Mental State Examination) was observed, there were no signs of lower cranial nerve palsy, limb motor or sensory deficits, ataxia, or gait abnormalities. Head magnetic resonance imaging (MRI) noted a mass lesion in the frontal lobe (at the right middle frontal gyrus with 26 mm diameter, at the left straight gyrus with 5 mm diameter) with clear margins and surrounding edematous changes. MRI indicated the lesion in the form of a high-intensity mass on diffusion weighted image (Figure 1A) and low intensity mass on flow attenuated inversion recovery (FLAIR) imaging (Figure 1B). Gadolinium contrast-enhanced MRI showed a ring-like enhanced contrast image (Figure 1C). Positron emission tomography showed that both methionine (Figure 1D) and 5-fluoro-deoxy-glucose (data not shown) accumulated in the lesion. Serum test marked increased interleukin 2 receptor (IL-2R).
Under general anesthesia, the right mass lesion was identified and biopsy was performed under navigation-guided, rigid endoscopic view. The interior of the mass was milky white and sol-like. Pathological examination revealed positivity for cluster of differentiation (CD) 20, B-cell/chronic lymphocytic leukemia (CLL) lymphoma (bcl) 2, and negativity for CD10, CD3, CD5, glial fibrillary acidic protein (GFAP), confirming a diagnosis of diffuse large B cell lymphoma.
He was treated with high-dose methotrexate-based chemotherapy (methotrexate 3.5 g/m2, procarbazine 150 mg/day, and vincristine 2 mg) 6 times and high-dose methotrexate-based chemotherapy with Rituximab 3 times. The patient also underwent radiation therapy with stereotactic radiotherapy (SRT) at 20 Gray in 2 fractions against the right frontal lobe lesion.
However, the lesion and the surrounding edema did not shrink (partial response: PR). The edema in the right frontal lobe became severe with mid line shift (Figure 2). On the other hand, the patient was also suffering from left-sided paralysis and psychiatric symptoms (restlessness and risk-taking behavior), which made it difficult to continue intravenous infusion therapy in the hospital.
Therefore, after careful explanation of the disease to the family and obtaining their consent, the patient was treated with only TMZ (150 mg/m2 for 5 days, 23 days rest) as an outpatient for 29 sessions, continuously. After 29 months of TMZ therapy, chemotherapy was stopped, and the patient has not had any recurrence since then.
The head MRI showed the diminished lesion and surrounding edema (Figure 3).
Discussion
The author has described a case of an elderly patient with PCNSL who failed standard therapy, but who responded well to TMZ as an alternative therapy. Temozolomide is a water-soluble imidazotetrazinone that exhibits excellent antineoplastic properties. It is an orally bioavailable alkylating agent able to penetrate the blood–brain barrier due to its lipophilic nature. Temozolomide functions by modifying DNA or RNA through the addition of methyl groups (alkylation). This causes substitution of thymine for cytosine during DNA replication, which creates a mismatched base pair. The transferred methyl group by TMZ can be removed by O6-methylguanine-DNA methyltransferase (MGMT). Although TMZ has highly active antineoplasm properties, adverse events from oral administration of that is usually mild. It is different from methotrexate in that it does not risk of causing severe injury to the kidneys, allowing for continuous anticancer therapy without interruption due to side effects.
Oral TMZ administration therapy does not require hospitalization and can be continue on an outpatient basis. In addition, TMZ is associated with fewer side effects than methotrexate or other anticancer agents. Temozolomide-based chemotherapy may help facilitate a change to home care in elderly patients.
However, using TMZ for chemotherapy in PCNSL is considered an off-label treatment in Japan in 2024. In addition, the efficacy of TMZ in combination with methotrexate was not proven in the Japan Clinical Oncology Group (JCOG) [8]. Methotrexate-based chemotherapy has been used in many cases with good response rates. The add-on effect of TMZ to methotrexate-based chemotherapy could not be adequately demonstrated in those clinical trials. Although there is a lack of accumulated case series reports, a few case reports of TMZ-based chemotherapy confirm its efficacy [8] [9] [10]. This case may suggest that TMZ alone may be effective in the treatment of patients with PCNSL who have failed to respond to methotrexate-based chemotherapy. Elderly patients and renal dysfunction are negative factors for the implementation and efficacy of methotrexate-based chemotherapy, which often do not provide adequate therapeutic response. The findings in this case may suggest that TMZ-based chemotherapy could be considered as salvage therapy for methotrexate-based chemotherapy.
However, further studies and comparisons with other treatment methods would be necessary to provide sufficient medical evidence. Less invasive combination pattern for each therapeutic ways was discussed [11], [12] More accumulation of case results and well discussions should be required to make next generation strategy against PCNSL.
Conclusion
Oral TMZ administration was effective in an elderly patient with PCNSL, who had failed standard chemotherapy and radiation therapy. It was less invasive, allowed for transition to outpatient management, and provided adequate therapeutic benefit. Future indications for similar cases should be considered. This case contains a rare experience of long-term treatment with TMZ. Over two years, TMZ administration had no severe adverse events with sufficient tumor suppression effects.
REFERENCES
1.
Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: A surveillance, epidemiology, and end results analysis. Cancer 2002;95(7):1504–10. [CrossRef]
[Pubmed]

2.
Schaff LR, Grommes C. Primary central nervous system lymphoma. Blood 2022;140(9):971–9. [CrossRef]
[Pubmed]

3.
Villanueva G, Guscott M, Schaiquevich P, et al. A systematic review of high-dose methotrexate for adults with primary central nervous system lymphoma. Cancers (Basel) 2023;15(5):1459. [CrossRef]
[Pubmed]

4.
Fritsch K, Kasenda B, Schorb E, et al. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 2017;31(4):846–52. [CrossRef]
[Pubmed]

5.
Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: Final results and long-term outcome. J Clin Oncol 2013;31(31):3971–9. [CrossRef]
[Pubmed]

6.
Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11(11):1036–47. [CrossRef]
[Pubmed]

7.
Poortmans PMP, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol 2003;21(24):4483–8. [CrossRef]
[Pubmed]

8.
Mishima K, Nishikawa R, Narita Y, et al. Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C. Neuro Oncol 2023;25(4):687–98. [CrossRef]
[Pubmed]

9.
Chiesa S, Hohaus S, Falcinelli L, et al. Chemoradiotherapy with temozolomide after high-dose methotrexate for primary CNS lymphoma: A multicenter phase I study of a response-adapted strategy. Ann Hematol 2020;99(10):2367–75. [CrossRef]
[Pubmed]

10.
Faivre G, Butler MJ, Le I, Brenner A. Temozolomide as a single agent maintenance therapy in elderly patients with primary CNS lymphoma. Clin Lymphoma Myeloma Leuk 2019;19(10):665–9. [CrossRef]
[Pubmed]

11.
Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 2007;25(30):4730–5. [CrossRef]
[Pubmed]

12.
Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015;125(9):1403–10. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Akira Tempaku - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthor declares no conflict of interest.
Copyright© 2025 Akira Tempaku. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.