|
Case Report
Neurological immune-related toxicity to ipilimumab/nivolumab therapy in malignant melanoma: A challenging case report
1 Doctor, Medical Oncology Department, Instituto Português de Oncologia do Porto Francisco Gentil E.P.E., Porto, Portugal
2 Doctor, Neurology Department, Instituto Português de Oncologia do Porto Francisco Gentil E.P.E., Porto, Portugal
3 Doctor, Infectious Diseases Department, Instituto Português de Oncologia do Porto Francisco Gentil E.P.E., Porto, Portugal
Address correspondence to:
Diana Baptista da Mata
Rua Dr. António Bernardino de Almeida 865, 4200-072 Porto,
Portugal
Message to Corresponding Author
Article ID: 100135Z10DM2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
da Mata DB, Rodrigues PR, Oliveira V, Santos FV, Ferreira P. Neurological immune-related toxicity to ipilimumab/nivolumab therapy in malignant melanoma: A challenging case report. J Case Rep Images Oncology 2024;10(2):10–14.ABSTRACT
Introduction: Malignant melanoma poses a significant threat due to its aggressiveness and high fatality rates. Recent advances in immunotherapy and targeted molecular therapies have transformed the treatment landscape for advanced melanoma, improving overall survival. However, the rising use of immune checkpoint inhibitors (ICIs) introduces complexities, particularly in neurological immune-related adverse events (irAEs), necessitating careful consideration and multidisciplinary management.
Case Report: We report the case of a 77-year-old woman with stage IV cutaneous melanoma, which was hospitalized with polyneuropathy following immunotherapy treatment with ipilimumab and nivolumab combination. The complex presentation involved considerations of COVID-19 vaccination, detection of Epstein–Barr virus (EBV) in cerebrospinal fluid, and the challenge of distinguishing infectious from immune-related causes. The multidisciplinary team navigated multiple diagnostic uncertainties. The patient’s clinical evolution, including complications and treatment responses, provided insights into managing severe neurological irAEs associated with immunotherapy.
Conclusion: Neurologic immune-related (IR) toxicity is well established, and it may led to severe adverse events, which are complex to diagnose and manage. This case report highlights the challenges and difficulties found, with relevant confounding factors, which arise the relevance of the need of a multidisciplinary team to deal with neurologic immune-related toxicities.
Keywords: Immune-related adverse events, Immunotherapy, Melanoma, Polineuropahty
Introduction
Malignant melanoma (MM) comprises less than 10% of all cutaneous cancers [1]. Nevertheless, due to its aggressive behavior, it is responsible for most deaths within these types of malignancies [2]. Prognosis varies depending on the disease stage at the moment of diagnosis [3]. The treatment for advanced/metastatic MM has changed deeply throughout the past years, with the development of immunotherapy and targeted molecular therapies [2].
These changes in MM treatment brought visible benefits, demonstrated through a clear rising in overall survival (OS) [4],[5],[6].
As the use of immune checkpoint inhibitors (ICIs) has increased, their toxicities have become a challenge in clinical practice. Neurological immune-related adverse events (irAEs) have been reported with an estimated incidence of 1–5% [7]. These irAEs may affect the central (CNS) or peripheral nervous system (PNS). It is extremely important to be aware of irAEs and evaluate these patients in a multidisciplinary team, in order to provide the best possible care.
In this article, we report a multidisciplinary case of stage IV cutaneous melanoma treated with an immunotherapy combination and admitted to the hospital due to newly diagnosed polyneuropathy.
Case Report
We present a case of a 77-year-old woman with Eastern Cooperative Oncology Group performance status (ECOG-PS) 1. She had essential thrombocythemia (treated with hydroxyurea and well-controlled) and hypothyroidism (treated with levothyroxine and also controlled) as main comorbidities. In 2015, the patient was diagnosed with cutaneous melanoma (classified as stage I, according to the American Joint Committee on Cancer (AJCC) staging) with a present BRAF V600E mutation and underwent local excision, followed by clinical surveillance. In 2019, the patient was diagnosed with axillary lymph node recurrence and underwent excision.
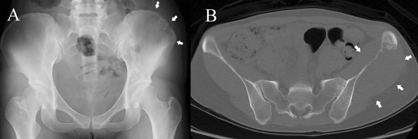
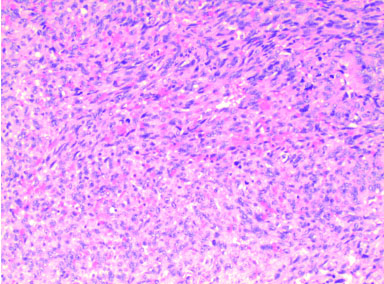
In November 2020, there was evidence of disseminated disease, with lung, lymph node, and subcutaneous metastasis, histologically confirmed (Figure 1).
Treatment with encorafenib and binimetinib (oral administration, 450 mg once a day and 45 mg twice a day, respectively) was initiated. This therapy was stopped in August 2021 due to disease progression.
She started second-line treatment with ipilimumab (3 mg/kg, every 21 days) associated with nivolumab (1 mg/kg, every 21 days) in October 2021 and completed three cycles on November 29, 2021.
On December 17, 2021, the patient presented to the Emergency Department with progressive ascending numbness, paresthesia, severe neuropathic pain of the lower limbs (up to her knees at the time of hospital admission), and weakness with progressive difficulty walking, all evolving over the course of one month.
It is noteworthy that she had received the COVID-19 vaccine two weeks before the onset of symptoms.
The physical examination revealed a subcutaneous left dorsal lesion (~4 cm), associated with multiple 2 cm erythematous lesions. The neurological examination showed symmetric paraparesis with distal predominance (with a Medical Research Council muscular strength of 4/5 in knee flexion/extension, 3/5 in dorsiflexion, and 2/5 in plantar flexion and finger flexion/extension), lower limb areflexia, hypoesthesia in a stocking-glove pattern up to the knees and wrists, hypopallesthesia in the lower limbs up to iliac crest level, and impaired joint position sensation at the toes. The patient was admitted for study. Blood analysis was performed at admission and is represented in Table 1.
Cerebrospinal fluid (CSF) analysis revealed a high leukocyte count, predominantly composed of lymphocytes, with high protein level. Cytology for malignant cells was negative. Cerebrospinal fluid microbiological studies were negative (bacteriological and mycobacteriological cultures, detection of cytomegalovirus (CMV), Varicella-zoster virus (VZV), herpes simplex virus 1/2 (HSV-1/2) by polymerase chain reaction (PCR) except Epstein–Barr virus (EBV), which was positive. It is important to note that she had positive EBV serology for IgG and negative for IgM, compatible with previous contact. All other systemic screenings were negative, including blood cultures and serologies for human immunodeficiency virus (HIV) and hepatitis virus B and C.
Electromyography revealed marked sensory-motor axonal and demyelinating polyneuropathy with signs of denervation, and lumbar magnetic resonance imaging (MRI) described a thickening with signal enhancement of the roots of the cauda equina, compatible with an infectious or inflammatory etiology.
Cerebral MRI revealed four small lesions (with a maximum 9 mm diameter) with no significant edema associated (Figure 2). Fluorodeoxyglucose-positron emission tomography (FDG-PET) scan was also performed and showed a partial response on extra-cerebral disease.
During the hospitalization, daily fever was documented on the 5th day.
In summary, the patient had acute polyradiculoneuropathy and fever, with no other systemic symptoms, with CSF pleocytosis and high protein count, but also a positive CSF EBV count.
At this time, the question arose: Was the EBV virus just an epiphenomenon of an immune polyneuropathy, or could it be the cause of an acute infectious polyradiculoneuropathy, which although rare, could be possible? Also, the role of COVID-19 vaccination was considered as a possible trigger for an immune-mediated condition, but after discussion within a multidisciplinary team (composed of a neurologist, an infectious diseases specialist, and a medical oncologist), we concluded that this event was not the main cause for the clinical presentation of this patient.
Even considering the main hypothesis of an immune checkpoint inhibitor-related peripheral neuropathy, treatment with corticosteroids was postponed since an infectious cause couldn’t be excluded. So, intravenous immunoglobulins (IvIg, 0.4 mg/kg/day for 5 days) and intravenous acyclovir (10 mg/kg 8/8 h for 14 days) were started.
Eight days after starting the aforementioned therapy, the patient was not showing any favorable neurological response to the treatment, while also maintaining high temperatures (without another infectious cause). A new CSF analysis was performed, once again showing an elevated lymphocytic count and a high protein level. However, EBV analysis was negative at this time. Table 1 shows comparative results from CSF analysis.
At this point, the multidisciplinary team decided to start high-dose steroid therapy (prednisone 1 mg/kg/day), considering the probable diagnosis of an immune checkpoint inhibitor-related neuropathy with no response to IvIg, and the low probability of an infectious cause (EBV related).
With optimization of physical rehabilitation, the patient started to show clinical improvement and was discharged from the hospital on January 12, 2022. After one month of high-dose prednisolone, it was possible to slowly taper the steroids over the course of four months.
The case was discussed in the Multidisciplinary Oncology Group, and due to this serious adverse event, the decision to permanently stop systemic treatment was taken. In March 2022, the patient underwent radiosurgery for brain lesions.
In September 2022, the patient had recovered from most neurological deficits, being able to walk on her own, maintaining only a distal lower limb hypoesthesia, up to her ankles. She maintained an ECOG PS 1. However, both cerebral and extra-cerebral disease progression was documented.
A rechallenge with dabrafenib and trametinib (oral administration, 150 mg twice a day and 2 mg once a day, respectively) was started and maintained until March 2023, when disease progression occurred. The patient died on June 25, 2023.
Discussion
Neurologic immune-related (IR) toxicity is well established, and it is recognized that immunotherapy combinations are more likely to cause severe adverse events than monotherapy [8]. The occurrence of a Guillain–Barré-like syndrome is a rare manifestation of IR peripheral neuropathy, with an incidence of less than 0.5% [7]. In such cases, corticosteroid treatment is generally associated with a favorable outcome, in contrast to non-ICI Guillain–Barré syndrome, where intravenous immunoglobulin is the preferred treatment [9].
This case holds particular significance due to several clinical confounders. The detection of Epstein–Barr virus (EBV) in the spinal fluid added complexity to the diagnostic pathway. The clinical significance of detecting EBV in central nervous system (CNS) infections remains unclear. In many reported cases of CNS infection, EBV was commonly found alongside other pathogens [10]. While the likelihood of an actual clinical EBV reactivation is low and could be a coincidental finding of no clinical significance (an epiphenomenon triggered by an immunological mechanism), this confounder delayed the initiation of corticosteroid treatment.
Considering the recent and prior COVID-19 vaccination of the patient, severe and unexpected post-vaccination neurological complications are rare but have been documented after COVID-19 vaccination, including Guillain–Barré syndrome, facial palsy, other neuropathies, encephalitis, meningitis, myelitis, autoimmune disorders, and cerebrovascular events [11]. Moreover, a potential association between COVID-19 vaccines and herpesvirus reactivation exists. Evidence for Varicella-zoster virus (VZV) and herpes simplex virus (HSV) has been supported by observational studies. However, for other herpesviruses like EBV and cytomegalovirus (CMV), further research is needed to understand the interaction between COVID-19 vaccination and their reactivation [12].
The presence of brain metastases was not considered a contributing factor to the clinical findings.
The positive clinical response to high-dose corticosteroid treatment provided stronger evidence that the patient likely experienced severe neurological immune-related adverse events (irAEs) due to ipilimumab/nivolumab treatment. European recommendations advise discontinuing immune checkpoint inhibitors (ICI) in grade 3 or 4, associated with severe symptoms, aligning with the actions taken in this case [7].
Conclusion
This case report brings attention to the nuances of neurological immune-related adverse events (irAEs). Though uncommon, the diagnosis of these effects poses considerable challenges. The key to their impact lies in early detection, a factor that significantly improves prognosis.
Moreover, the narrative underscores the imperative of a multidisciplinary approach. Given the immunotherapy’s tendency to mirror autoimmune diseases, it becomes evident that collaborative expertise is crucial. Medical oncologists, while adept in their domain, may find themselves outside their comfort zone, emphasizing the critical need for a diverse and collaborative approach in managing such complex cases.
REFERENCES
1.
Michielin O, van Akkooi A, Lorigan P, et al. ESMO consensus conference recommendations on the management of locoregional melanoma: Under the auspices of the ESMO Guidelines Committee. Ann Oncol 2020;31(11):1449–61. [CrossRef]
[Pubmed]

2.
Radiation: Ultraviolet (UV) radiation and skin cancer. World Health Organization. 2017. [Available at: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer

3.
Michielin O, van Akkooi ACJ, Ascierto PA, et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30(12):1884–901. [CrossRef]
[Pubmed]

4.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381(16):1535–46. [CrossRef]
[Pubmed]

5.
Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372(1):30–9. [CrossRef]
[Pubmed]

6.
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372(26):2521–32. [CrossRef]
[Pubmed]

7.
Haanen J, Obeid M, Spain L, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33(12):1217–38. [CrossRef]
[Pubmed]

8.
Ma X, Zhang Y, Wang S, Wei H, Yu J. Immune checkpoint inhibitor (ICI) combination therapy compared to monotherapy in advanced solid cancer: A systematic review. J Cancer 2021;12(5):1318–33. [CrossRef]
[Pubmed]

9.
Haugh AM, Probasco JC, Johnson DB. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf 2020;19(4):479–88. [CrossRef]
[Pubmed]

10.
Lee GH, Kim J, Kim HW, Cho JW. Clinical significance of Epstein-Barr virus in the cerebrospinal fluid of immunocompetent patients. Clin Neurol Neurosurg 2021;202:106507. [CrossRef]
[Pubmed]

11.
Tondo G, Virgilio E, Naldi A, Bianchi A, Comi C. Safety of COVID-19 vaccines: Spotlight on neurological complications. Life (Basel) 2022;12(9):1338. [CrossRef]
[Pubmed]

12.
Shafiee A, Amini MJ, Arabzadeh Bahri R, et al. Herpesviruses reactivation following COVID-19 vaccination: A systematic review and meta-analysis. Eur J Med Res 2023;28(1):278. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Diana Baptista da Mata - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Patrícia Rafaela Rodrigues - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Vanessa Oliveira - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fábio Videira Santos - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Paula Ferreira - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Diana Baptista da Mata et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.