|
Case Report
A case report of a rare endocervical-type typical polypoid adenomyoma
1 Department of Obstetrics and Gynecology, Guangyuan Central Hospital, Guangyuan, Sichuan, China
2 Department of Pathology, Guangyuan Central Hospital, Guangyuan, Sichuan, China
Address correspondence to:
Guang-Zong Zhao
Guangyuan Central Hospital, No. 16 Jing Alley, Lizhou District, Guangyuan City, Sichuan,
China
Message to Corresponding Author
Article ID: 100126Z10HH2023
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
He H, Rouzi N, Chen T-A, Hou Y, Zhang L, Zhao G-Z. A case report of a rare endocervical-type typical polypoid adenomyoma. J Case Rep Images Oncology 2023;9(2):19–23.ABSTRACT
Introduction: Endocervical-type typical polypoid adenomyoma is an exceptionally rare benign tumor, which should be seldom reported to date. We aim to share our clinical experience about endocervical-type typical polypoid adenomyoma and review relevant publications to decrease the rates of misdiagnosis and missed diagnosis.
Case Report: A 15-year-old adolescent had recurrent irregular vaginal bleeding persisting for six months, and the vaginal mass could not be returned after toileting finally. Ultrasound examination revealed a huge mass with heterogeneous hypoechogenicity and prominent intramural blood flow, comprising multiple cystic areas. Magnetic resonance imaging demonstrated significant endometrial thickening, and a well-defined pedunculated mass of varying signal intensity protruding into the vaginal orifice. Hysteroscopy was performed, and a pathologic biopsy of the mass was conducted. Pathological analysis revealed an endometrial epithelial monolayer overlaying the tissue, with subtle glandular hyperplasia and partial gland expansion. The stroma consisted of fibrous connective tissue with a few smooth muscle fibers.
Conclusion: The prolapsed mass was confirmed as an endocervical-type typical polypoid adenomyoma. We successfully eradicated the tumor through hysteroscopy, leading to the restoration of normal cervical morphology, correction of anemia, and normalization of body temperature. During the procedure, we directly observed the pink mass, exhibiting cystic changes, encircling the hypertrophied cervix. Persistent exposure to high-dose estrogen may potentially play a significant role in the development of polypoid adenomyoma.
Keywords: Adenomyoma, Adolescent, Hysteroscopy, Vaginal bleeding
Introduction
Uterine polypoid adenomyomas, also known as adenomyomyoid polyps, are relatively rare in the clinic. The pathological features of uterine polypoid adenomyomas are that the polyp gland is surrounded by a large number of smooth muscle fibers rather than the normal polyp stroma. According to the type of glandular epithelium, it can be divided into two types: typical polypoid adenomyoma (TPA) and atypical polypoid adenomyoma (APA) [1],[2]. Although uterine polypoid adenomyoma has been referred widely in the literature, typical polypoid adenomyoma in the cervix, containing endometrioid-type glands, has been occasionally mentioned and discussed previously [3],[4],[5]. In addition, due to its uncommon occurrence, it is often underdiagnosed or misdiagnosed, thus delaying treatment.
In this report, we present a unique case of a 15-year-old adolescent with recurrent irregular vaginal bleeding, who was ultimately diagnosed with endocervical-type typical polypoid adenomyoma, in the absence of tamoxifen and hormone therapy. To the best of our knowledge, this is the first case report of a patient in the youngest age of onset. We aim to share our clinical experience about endocervical-type typical polypoid adenomyoma and review relevant publications to decrease the rates of misdiagnosis and missed diagnosis.
Case Report
A 15-year-old patient, gravida 0, para 0, had irregular vaginal bleeding, and the amount of menstruation was normal, without dysmenorrhea. Six months before admission, the patient was able to touch the vaginal orifice mass (approximately 2 cm) during toileting. However, the prolapsed mass could only be returned in the supine position or standing position. In addition, after forced defecation, she had irregular vaginal bleeding that lasts for about two days and loses fresh blood similar in volume to those as during common menstruation. On the day before admission, symptoms were exacerbated and the prolapsed mass could not be returned after toileting, with abnormal increments of vaginal bleeding and elevated body temperature (38.5°C). By admission, she had very low hemoglobin level (57 g/L) due to the blood-loss anemia. The vaginal mass was pedunculated and composed of two parts. One part appeared light pink and was located on the superior side, while the other part exhibited a purplish-black color with an unpleasant odor, which may be the primary cause of the fever.
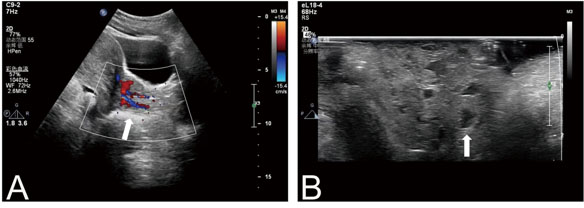
Transabdominal ultrasound revealed that the endometrial bilayer had an approximate thickness of 23 mm, exhibiting a heterogeneous echo with small cyst-like echogenicity. Furthermore, the solid mass displayed an inhomogeneous hypoechogenicity, with abundant blood flow signals observed internally (Figure 1A). The mass consisted of multiple cystic areas measuring 87×31 mm (Figure 1B) and could be traced from the cervical tube to the body surface, suggesting a possible origin from the endometrium. However, determining the exact derivation of the mass proved challenging. Consequently, we conducted further examination of the lesion using magnetic resonance imaging (MRI).
An MRI examination revealed evident thickening of the endometrium, along with a well-defined pedunculated mass exhibiting inhomogeneous intensity and protruding into the vaginal orifice. Sagittal T2-weighted images displayed a cordlike hyperintensity, possibly originating from the cervix or uterine cavity, connecting irregular and perplexing clump signals that entirely occupied the lower reproductive tract. Within the vaginal canal, the prolapsed mass divided into two parts, each displaying multiple cystic hyperintensities surrounded by a grid-like pattern of isointense signals (Figure 2A). Furthermore, these hyperintense features on T2-weighted images remained unattenuated on fat-suppressed T2-weighted imaging (Figure 2B). Additionally, axial post-contrast T1-weighted imaging revealed T1-hyperintense signals, indicating the presence of vascularity (Figure 3A). Notably, the uterine cavity exhibited hyperproliferative endometrium without a distinct demarcation line between the mass and the endometrium. This enhancement was observed on coronal post-contrast T1-weighted imaging (Figure 3B). To establish their relationship, hysteroscopy was performed.
The patient underwent hysteroscopy in the lithotomy position. We directly observed that the stalk of the pink mass, performing cystic changes, grew around the hypertrophy cervix. Due to the presence of tissue filling the cervical ostium, accessing the uterine cavity during hysteroscopy was challenging prior to the surgery. The mass was carefully pulled caudally and rotated using tissue forceps. After removing major tissue, the remained mass was completely cut away along the peduncle through hysteroscopy, with the cervical morphology returning to normal. Grossly, the pedunculated mass presented florid appearance, with a rough surface measuring approximately 80×36 mm. Then, images of uterine cavity were obtained under the hysteroscopy, while the endometrium was extensively thickened and looked rough with small cystic changes. The hysteroscopic finding verified that the mass indeed derived from cervix rather than uterine cavity. To further identify the vaginal mass, pathologic biopsy was conducted. Hematoxylin and eosin (HE) staining demonstrated that the tissue was overlaid by an endometrial epithelial monolayer, with unapparent gland hyperplasia and partial gland expansion. The stroma is composed of fibrous connective tissue and a few smooth muscle fibers (Figure 4).
Discussion
Typical polypoid adenomyoma (TPA) is a benign space-occupying lesion originating from the uterine cavity, with extremely rare cases reported in the uterine cervix, making them infrequently encountered in clinical practice [6]. Generally, the symptoms of TPA and APA, inclusive of menorrhagia, vaginal mass, anemia, and abnormal bleeding are nonspecific. Furthermore, there is no significant disparity in the diagnostic performance of ultrasound and MRI [7],[8]. Therefore, distinguishing between TPA and APA poses a challenge. Sajjad et al. pointed out that prolapsed uterine tissue, with blood-containing cystic spaces on MRI, should be considered as typical polypoid adenomyoma [9]. However, this viewpoint lacks persuasiveness. The histopathological staining still remains the most effective method for distinguishing between TPA and APA. Empirically, APA exhibited characteristic lesions under microscopic observation. Hyperplasia disordered glands frequently show branch-like or lobular arrangement, which are similar to endometrial complex atypical hyperplasia [10]. Concisely, glandular epithelial cells have mild and moderate atypicality, accompanied by squamous metaplasia. The gland may appear as differentiated adenocarcinoma-like structures in focal lesions. Finally, the prolapsed mass was confirmed as TPA through histopathological staining. The treatment of polypoid adenomyomas includes curettage, hysterectomy, and hysteroscopic resection. Curettage is an invisible operation that is hampered by its residual lesions and high recurrence rate in the clinic. Although hysterectomy can completely eradicate the lesion, it has a large trauma range and serious complications, which is just suitable for postmenopausal or no fertility intention patients with atypical polypoid adenomyomas. Hysteroscopy is a multifunctional tool that integrates imaging and treatment capabilities. It enables visible lesion removal, providing advantages such as accurate localization, complete resection, minimal tissue trauma, and a low recurrence rate. These results underscore the irreplaceable value of hysteroscopy in the diagnosis of location for polypoid adenomyomas when compared to ultrasound and MRI. Consequently, hysteroscopic resection should be recommended as the preferred treatment modality for young patients seeking fertility preservation [11].
The pathogenesis and mechanism of TPA have not received extensive research attention to date. However, there are scholars who propose that polypoid adenomyomas could potentially arise from endometrial stromal progenitor cells. These cells possess the ability to differentiate into smooth muscle cells, a process that is potentially triggered by prolonged exposure to estrogen [12]. Perimenopausal women experience a decline in estrogen levels, which has significant effects on the hypothalamic feeding centers and neurons. These effects result in a range of metabolic changes, including a decrease in metabolic rate, an increase in appetite, and the accumulation of fat in visceral organs. These alterations can potentially lead to increased estrogen synthesis and disturbances in glucose-lipid metabolism. Moreover, the decline in ovarian function, coupled with factors such as obesity-induced insulin resistance, can contribute to ovulation disorders. Consequently, these factors contribute to a sustained single estrogen action on the endometrium [13]. For postmenopausal women, several cases reported that patients diagnosed with adenomyomyoid polyps received treatment with tamoxifen for breast cancer [14],[15]. Tamoxifen is a class of agents known as selective estrogen receptor modulators (SERMs), which have both antiestrogen effects and estrogen-like effects on different tissues and organs [14]. In contrast to the antagonistic estrogen effect on the mammary gland, tamoxifen shows estrogen-like action on female reproductive organs. Therefore, tamoxifen may cause benign or malignant lesions, such as endometrial hyperplasia, endometrial polyps, endometrial cancer and uterine sarcoma. Adolescents who are within 2–3 years of their first menstruation (postmenarche) experience endometrial stimulation due to the continuous production of estrogen without the counterbalancing effect of progesterone. This occurs because their hypothalamic-pituitary-ovarian (HPO) axis is still immature [16]. Consequently, adolescent girls are susceptible to dysfunction caused by excessive mental stress, malnutrition, and anxiety, which can disrupt the regulation of the HPO axis and result in vaginal bleeding. Therefore, when formulating treatment plans, it is crucial to consider individualized and effective approaches that prioritize safety, fertility needs, and psychological and social ethical factors following surgery. Wiktor et al. reported a woman incidentally diagnosed with polypoid adenomyoma who received prolonged combination therapy for irregular, heavy abnormal uterine bleeding from the age of 15 [17]. Hence, we speculated that persistent high-dose estrogen may be an important factor in inducing polypoid adenomyoma. This conjecture can also be evidenced by the fact that the patient had abnormal endometrial thickening, with biopsy-proven endometrial hyperplasia and small foci of complex hyperplasia.
Conclusion
We described one case in which an adolescent suffered from a huge vaginal mass that was confirmed as an endocervical-type typical polypoid adenomyoma. We successfully eradicated the tumor, and directly observed the pink mass, exhibiting cystic changes, encircling the hypertrophied cervix through hysteroscopy. We surmise that persistent exposure to high-dose estrogen may potentially play a significant role in the development of polypoid adenomyoma. As the environment changes and social stresses increase, the incidence of polypoid adenomyoma may present a growing and younger trend.
Hua He and Nuermanguli Rouzi contributed equally to this work.
REFERENCES
1.
Huang C, Hong MK, Ding DC. Endometrial adenomyoma polyp caused postmenopausal bleeding mimicking uterine malignancy. Gynecol Minim Invasive Ther 2017;6(3):129–31. [CrossRef]
[Pubmed]

2.
Strickland KC, Yuan L, Quade BJ, Nucci MR, Howitt BE. Clinicopathological and immunohistochemical features of uterine adenomyomatous polyps. Hum Pathol 2019;84:239–45. [CrossRef]
[Pubmed]

3.
Wang X, Guo Y. Clinical analysis of 44 cases of atypical polypoid adenomyoma of the uterus. BMC Womens Health 2022;22(1):60. [CrossRef]
[Pubmed]

4.
Grindstaff S, Banet N. Atypical polypoid adenomyoma. Int J Gynecol Cancer 2021;31(4):639–40. [CrossRef]
[Pubmed]

5.
Takeda Y, Araki D, Arase T, Tsutsumi Y. Polypoid adenomyoma of endocervical type. Case Rep Pathol 2014;2014:275421. [CrossRef]
[Pubmed]

6.
Ota S, Ushijima K, Nishio S, et al. Polypoid endocervical adenomyoma: A case report with clinicopathologic analyses. J Obstet Gynaecol Res 2007;33(3):363–7. [CrossRef]
[Pubmed]

7.
Lee EJ, Joo HJ, Ryu HS. Sonographic findings of uterine polypoid adenomyomas. Ultrasound Q 2004;20(1):2–11. [CrossRef]
[Pubmed]

8.
Kitajima K, Imanaka K, Kuwata Y, Hashimoto K, Sugimura K. Magnetic resonance imaging of typical polypoid adenomyoma of the uterus in 8 patients: Correlation with pathological findings. J Comput Assist Tomogr 2007;31(3):463–8. [CrossRef]
[Pubmed]

9.
Sajjad N, Iqbal H, Khandwala K, Afzal S. Polypoid adenomyoma of the uterus. Cureus 2019;11(2):e4044. [CrossRef]
[Pubmed]

10.
Lu B, Yu M, Shi H, Chen Q. Atypical polypoid adenomyoma of the uterus: A reappraisal of the clinicopathological and immunohistochemical features. Pathol Res Pract 2019;215(4):766–71. [CrossRef]
[Pubmed]

11.
Chiyoda T, Lin BL, Saotome K, Kiyokawa S, Nakada S. Hysteroscopic transcervical resection for atypical polypoid adenomyoma of the uterus: A valid, fertility-preserving option. J Minim Invasive Gynecol 2018;25(1):163–9.e1. [CrossRef]
[Pubmed]

12.
Lee EJ, Han JH, Ryu HS. Polypoid adenomyomas: Sonohysterographic and color Doppler findings with histopathologic correlation. J Ultrasound Med 2004;23(11):1421–9. [CrossRef]
[Pubmed]

13.
Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol 2016;34(35):4225–30. [CrossRef]
[Pubmed]

14.
Ugwumadu AH, Bower D, Ho PK. Tamoxifen induced adenomyosis and adenomyomatous endometrial polyp. Br J Obstet Gynaecol 1993;100(4):386–8. [CrossRef]
[Pubmed]

15.
Nasu K, Arima K, Yoshimatsu J, Miyakawa I. Adenomyomatous polyp of the uterus in a patient receiving tamoxifen. Jpn J Clin Oncol 1997;27(5):350–2. [CrossRef]
[Pubmed]

16.
Deligeoroglou E, Karountzos V, Creatsas G. Abnormal uterine bleeding and dysfunctional uterine bleeding in pediatric and adolescent gynecology. Gynecol Endocrinol 2013;29(1):74–8. [CrossRef]
[Pubmed]

17.
Szewczuk W, Szewczuk O, Czajkowski K, Grala B, Semczuk A. Immunohistochemical results and case report of an incidental finding of uterine polypoid adenomyoma after long-time therapy for metrorrhagia. Pathol Res Pract 2020;216(7):152998. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Hua He - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nuermanguli Rouzi - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ting-An Chen - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yu Hou - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ling Zhang - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guang-Zong Zhao - Conception of the work, Design of the work, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2023 Hua He et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.