|
Case Report
Non-islet cell tumor causing hypoglycemia: A paraneoplastic complication of hepatocellular carcinoma
1 Department of Internal Medicine, The Brooklyn Hospital Center, 121 Dekalb Avenue, Brooklyn, NY 11201, USA
Address correspondence to:
Samridhi Sinha
DO, Department of Internal Medicine, The Brooklyn Hospital Center, 121 Dekalb Avenue, Brooklyn, NY 11201,
USA
Message to Corresponding Author
Article ID: 100125Z10SS2023
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Sinha S, Ahluwalia A, Wasserman C. Non-islet cell tumor causing hypoglycemia: A paraneoplastic complication of hepatocellular carcinoma. J Case Rep Images Oncology 2023;9(2):15–18.ABSTRACT
Introduction: Hypoglycemia is a common complication in patients with diabetes who are using either insulin or oral hypoglycemic agents. In non-diabetic patient hypoglycemia is more rare and paraneoplastic syndrome should be considered if other causes have been excluded. Hepatocellular cancer is an example of a non-islet cell tumor that can cause hypoglycemia.
Case Report: Here we present a case of a 71-year-old male with history of metastatic hepatocellular cancer, treated hepatitis C infection, and human immunodeficiency virus (HIV). The patient was on active treatment with nivolumab. During admission he developed multiple episodes of hypoglycemia. The tests revealed decreased C-peptide and insulin levels suggesting non-islet cell etiology. The patient was initially treated with glucagon, and then started on steroids thereafter his episodes of hypoglycemia resolved. He continued to have stable disease while on immunotherapy, as well as no further hypoglycemia events while remaining on long term steroids.
Conclusion: This case illustrates an important example of non-islet cell tumor hypoglycemia in a patient with hepatocellular carcinoma (HCC) where immunotherapy is emerging as a promising treatment option and the use of steroids can interfere with treatment. In situations where tumor resection, debulking, or ablation cannot be done, treatment of choice is glucagon for acute episodes and steroids can be considered for long-term management.
Keywords: Big IGF-2, Non-islet cell tumor causing hypoglycemia, Paraneoplastic
Introduction
Non-islet cell tumor hypoglycemia (NICTH) is a rare but serious complication of the malignancy. Severe hypoglycemia can occur in a small percentage of patients with non-islet cell tumors [1]. Of these non-islet cell tumors, hepatocellular carcinomas (HCCs) are the second leading cause of NICTH accounting for 23% of all cases after the mesenchymal tumors which account for 45% of cases [2].
The proposed underlying pathology of non-islet cell tumors causing hypoglycemia is that these tumors produce large amounts of “big” IGF-2, which is a combination of mature IGF-2 and incompletely processed IGF-2, this then inhibits glucose release from the liver, thereby causing hypoglycemia. Concurrently there is an increased utilization of the glucose in the skeletal muscles, which worsens hypoglycemia in these patients and can drive glucose levels <55 mg/dL [3].
In patients presenting with NICTH, laboratory evaluation shows low serum glucose, insulin, c-peptide, and beta-hydroxybutyrate levels with negative screening for oral hypoglycemic agents such as sulfonylureas and meglitinides. If the laboratory evaluation shows low insulin, c-peptide, and beta-hydroxybutyrate during hypoglycemic episodes, glucagon should be given in doses of 0.5–1 mg of intramuscular. An increase in plasma glucose level of more than 25 mg/dL with glucagon administration indicates the presence of insulin like growth factor [3].
In patients with non-islet cell tumor causing hypoglycemia, the IGF-2 to IGF-1 ratio is usually elevated to more than 10 [3]. Diagnosis of non-islet cell tumor can still be made without IGF-1 and IGF-2 levels, based on laboratory evaluation showing low insulin, c-peptide, and beta-hydroxybutyrate during hypoglycemic episodes, and with improved of serum glucose levels by more than 25 mg/dL with the administration of glucagon [3].
The definite treatment of NICTH is tumor removal or debulking or endoscopic ablation. In patients who are not eligible for surgery, long-term steroids should be considered as a treatment modality [4]. This case report summarizes the resolution of fasting hypoglycemic episodes which occurred due to a paraneoplastic complication of moderately differentiated hepatocellular carcinoma after starting the patient on steroids.
Case Report
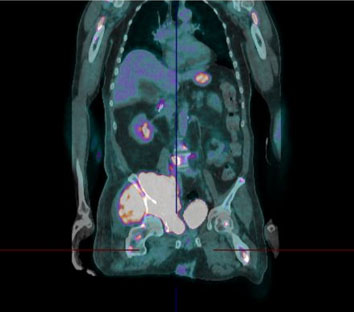
A 71-year-old male patient with past medical history of end-stage renal disease, hepatitis C, HIV, and moderately differentiated advanced metastatic hepatocellular cancer was presented. He was being treated with single agent Nivolumab immunotherapy. The patient was initially admitted for symptomatic anemia. After his admission he developed multiple episodes of hypoglycemia as low as 31 mg/dL. During these episodes the patient became confused, diaphoretic, and lightheaded. Vital signs were within normal limits. The patient was given 1 mg intramuscular glucagon, for the improvement of mental status and normalization of serum glucose levels to 167 mg/dL. The patient’s workup revealed no suspicious findings on brain imaging and exhibited no physical signs of acromegaly. Surreptitious insulin use was excluded as the patient had low levels of insulin on blood work. Cortisol battery test was within normal range. IGF-II levels could not be done at our institution. The patient did not have any signs of infection on chest X-ray, and blood cultures were negative. Thyroid function tests were within normal range. Computed tomography (CT) abdomen and pelvis showed innumerable lesions throughout the liver which were compatible with multifocal hepatocellular carcinoma, there were no pancreatic mass. The patient’s hypoglycemia was unrelated to his hemodialysis sessions, and the patient denied any loss of appetite or decreased portion size. Further laboratory tests revealed C-peptide 0.00437 ng/dL (0.80–3.85 ng/L), insulin 1.7 (=19.6 uIU/mL) suggesting non-islet cell etiology.
The patient continued to have recurrent episodes of hypoglycemia with neuroglycopenic symptoms which were initially treated with glucose tablets and glucagon. The patient was subsequently started on prednisone 2 mg every 12 hours and a frequent diet plan after which his hypoglycemic episodes resolved. The patient was continued on this dose of steroids and remained on nivolumab. During this time, he showed no signs of progression of disease and no further hypoglycemic episodes. Unfortunately, the patient passed away from COVID-19-related complications after five months of treatment for non-islet cell tumor causing hypoglycemia.
Discussion
Non-islet cell tumor hypoglycemia is a rare paraneoplastic endocrine syndrome. It can result from either the production of aberrant hormone precursors or hormone-like substances by tumors or the rapid growth of tumor in emaciated patients. The backbone of treatment often involves the use of steroids [5]. This case highlights this rarely seen syndrome within HCC, and the conundrum of how to manage patients with NICTH in the setting of immunotherapy use.
The recognition of paraneoplastic endocrine complications in patients is critical as it can be helpful for the diagnosis of a neoplasm or can inform prognosis in patients where a neoplasm is already established [3]. While all causes of hypoglycemia in HCC are not well understood there are two known theories for hypoglycemia to occur in the context of cancer patients. Firstly, type A NICTH is seen in emaciated patients with rapidly growing cancer. In these cases, much of the liver can be replaced with tumor impairing its ability to replenish blood glucose levels from glycogen. Type A hypoglycemia is more commonly seen in advanced HCC and is associated with suppressed insulin and c-peptide levels and increased glucagon levels. Type B NICTH involves the complex role of IGFII processing in the liver and represents 5–13% [5],[6],[7] of paraneoplastic hypoglycemia in HCC. Here hepatocytes are not able to process IGF2 precursors, resulting in the increase in circulating levels of the hormone. The IGF2 precursors are smaller molecules and can pass through capillary membranes easily, giving them increased access to IGF1, IGF2, and insulin receptors resulting in greater glucose uptake [5],[6],[7].
The typical pattern for NICTH is seen in our case which includes: low glucose (serum glucose <55 mg/dL) with simultaneous low insulin, proinsulin, C-peptide, hydroxybutyrate levels, and the absence oral hypoglycemic agent use [8],[9]. The next step in our patients’ diagnostic workup would have been to evaluated IGF levels. In the case of type B NICTH, IGF-II levels can be elevated or normal. In order to properly evaluate the levels IGF-I levels would also need to be evaluated and an elevation in IGF-II:IGF-I ratio above 3:1, this ratio can often increase to greater than 10:1 and is useful in helping to differentiating the diagnosis of NICTH [10],[11],[12],[13].
In our patient the tumor burden in the liver was high, almost 95% of the liver parenchyma was replaced by malignant tissues, making it unable to replenish blood glucose levels from glycogen, hence making our patient type A NICTH.
While the initial treatment of hypoglycemia is immediate correction of hypoglycemia by giving glucose or dextrose-containing fluids, treatment directed at underlying malignancy and once NICTH is identified and a primary tumor is found, the mainstay of treatment is a surgical resection [2]. In cases where surgical resection or debulking is not possible glucocorticoids (including dexamethasone, hydrocortisone, prednisolone, and prednisone, typically in doses equivalent to prednisone 30–60 mg/d) are the most extensively described medical therapy for NICTH [4]. In one case series 4/8 patients treated with steroids achieved and durable sustained responses to steroids (range 5–9 months) that were either maintained until death or still ongoing when the series was published [4].
Conclusion
Non-islet cell hypoglycemia is a rare and serious paraneoplastic syndrome in patients with hepatocellular carcinoma. Hypoglycemia in patients with hepatocellular carcinoma has been worked up diligently as the approach to treatment is multi-disciplinary. Treatment options range from local regional treatment of the malignancy to steroids for long-term management. Our case highlights the option of using maintenance steroids in patients with NICTH receiving immunotherapy and did not appear to adversely affect response to immunotherapy. This finding is a single case and further investigation is needed to draw more concrete conclusions.
REFERENCES
1.
Scott K. Non-islet cell tumor hypoglycemia. J Pain Symptom Manage 2009;37(4):e1–3. [CrossRef]
[Pubmed]

2.
Kahn CR. The riddle of tumour hypoglycaemia revisited. Clin Endocrinol Metab 1980;9(2):335–60. [CrossRef]
[Pubmed]

3.
4.
Teale JD, Wark G. The effectiveness of different treatment options for non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf) 2004;60(4):457–60. [CrossRef]
[Pubmed]

5.
Nikeghbalian S, Bananzadeh A, Yarmohammadi H. Hypoglycemia, the first presenting sign of hepatocellular carcinoma. Saudi Med J 2006;27(3):387–8.
[Pubmed]

6.
7.
Yeung RTT. Hypoglycaemia in hepatocellular carcinoma: A review. Hong Kong Med J 1997;3(3):297–301.
[Pubmed]

8.
Baxter RC. The role of insulin-like growth factors and their binding proteins in tumor hypoglycemia. Horm Res 1996;46(4–5):195–201. [CrossRef]
[Pubmed]

9.
Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol (Oxf) 1998;49(4):491–8. [CrossRef]
[Pubmed]

10.
Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res 2006;16(4):211–6. [CrossRef]
[Pubmed]

11.
Teale JD. Non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf) 1999;51(2):147. [CrossRef]
[Pubmed]

12.
Dynkevich Y, Rother KI, Whitford I, et al. Tumors, IGF-2, and hypoglycemia: Insights from the clinic, the laboratory, and the historical archive. Endocr Rev 2013;34(6):798–826. [CrossRef]
[Pubmed]

13.
Hizuka N, Fukuda I, Takano K, Okubo Y, Asakawa-Yasumoto K, Demura H. Serum insulin-like growth factor II in 44 patients with non-islet cell tumor hypoglycemia. Endocr J 1998;45 Suppl:S61–5. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Samridhi Sinha - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Amith Ahluwalia - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Carrie Wasserman - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2023 Samridhi Sinha et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.