|
Case Report
Spontaneous regression of squamous cell carcinoma of the lung with high PD-L1 expression: A case report and literature review
1 MBBS, Senior House Officer, Department of Radiation Oncology, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
2 BSc, MBBS, FRANZCR, Radiation Oncologist, Department of Radiation Oncology, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
3 BPharm, MD, FRANZCR, Radiation Oncologist, ICON Cancer Centre Greenslopes, Brisbane, QLD, Australia
Address correspondence to:
Kelleher Retchford
81 Homebush Rd, Kedron, 4031 QLD,
Australia
Message to Corresponding Author
Article ID: 100120Z10KR2023
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Retchford K, Bettington C, Newman SJ. Spontaneous regression of squamous cell carcinoma of the lung with high PD-L1 expression: A case report and literature review. J Case Rep Images Oncology 2023;9(1):17–21.ABSTRACT
Introduction: Lung cancer is the leading cause of cancer-related mortality worldwide. Squamous cell carcinomas (SCC) are responsible for a significant proportion of this burden. Spontaneous remission of cancer is a known phenomenon, albeit a rare event. The exact etiological mechanism(s) are not appreciated, although are largely predicated on the complex interactions between the malignant cells, tumor microenvironment and the immune system.
Case Report: We present the case of an incidentally found, biopsy proven lung SCC with high PD-L1 expression that underwent spontaneous regression (SR) prior to definitive therapy. The patient underwent computed tomography (CT)-guided biopsy of the lesion and coronary artery bypass grafting in the time preceding the tumor regression. The observed regression has been sustained during ongoing follow-up.
Conclusion: There are complex interactions between malignant cells, the tumor microenvironment, and the immune system, with evasion of immune destruction being a well-recognized and studied hallmark of cancer. There are multiple factors that may contribute to immune recognition of cancer and its subsequent regression and cases such as this highlight that there is much yet to elucidate. Further identification of these cases and their molecular characteristics will add to our understanding of the process. In the unknown lies the promise of improving cancer outcomes.
Keywords: Non-small cell lung cancer, PD-L1, Spontaneous regression, Squamous cell carcinoma
Introduction
Lung cancer is the leading cause of cancer mortality globally, with over 2.2 million new cases annually [1]. In Australia, only 22% of diagnosed individuals are alive at five years [2]. Squamous cell carcinoma (SCC) of the lung accounts for approximately 20% of all diagnosed lung cancers [3]. The treatment of lung cancer depends on disease stage and patient factors but typically involves some combination of surgery, radiotherapy, chemotherapy, and/or immunotherapy. Spontaneous regression (SR) of cancer, also termed spontaneous remission, is rare and has been estimated at rates from 1 in 60,000 to 1,000,000 cancers. Some malignancies such as melanoma, leukemia, and lymphoma have a higher frequency of SR, whereas in lung cancer it is comparatively rare [4],[5]. A recent review of published cases by Zhang et al. found 14 reported cases between 1988 and 2018 [5]. The mechanisms behind SR are not well understood. Here we present a case of an incidentally discovered, biopsy proven lung SCC undergoing SR in the absence of definitive oncologic therapy.
Case Report
In March 2020, a 76-year-old male underwent CT cervical spine for a planned right C3/4 and C4/5 foraminotomy. An incidental 17 mm spiculated lesion in the left upper lobe (LUL), suspicious for a primary lung malignancy, was identified. Clinical examination was only notable for bibasal crepitations. No hilar or mediastinal lymphadenopathy was found on subsequent CT chest (Figure 1).
His past medical history was notable for a solitary kidney due to pediatric trauma, hypertension, ischemic heart disease with myocardial infarctions (MI) at the age of 39 and 63 and chronic obstructive pulmonary disease with recent lung function tests of FEV1 2.30L (86%), FVC 3.90L (110%), and DLCO 67% predicted. The patient is a retired truck driver, crane operator, and salesman who lives with his wife. He is a 30-pack-year ex-smoker who quit at 65 years old. He rarely consumes alcohol.
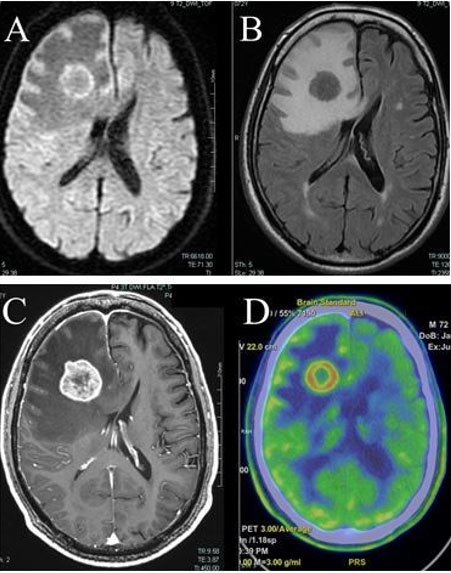
He proceeded to CT-guided core biopsy which confirmed SCC, with areas of necrosis and a PD-L1 tumor proportion score (TPS) of 65% (Roche Ventana SP263 antibody Benchmark Ultra assay). Staging FDG-PET/CT showed the LUL lesion to be intensely FDG avid, with a mild-moderately FDG avid ipsilateral station 10 lymph node, suspicious for clinical N1 disease (Figure 2). There was no extra-thoracic disease. It was designated clinical stage IIB cT1bN1M0 LUL SCC (AJCC/UICC 8th Edition).
During the diagnostic work up, he was hospitalized with acute pulmonary edema in the setting of coronary artery disease. His case was discussed at a quaternary center pulmonary malignancy multidisciplinary meeting (MDT) with a recommendation for a coronary artery bypass graft (CABG) in addition to a left upper lobectomy as definitive treatment for his lung SCC. If medically unsuitable for surgery, treatment with definitive external beam radiotherapy was recommended as an alternative. The patient successfully underwent CABG in September 2020; however, due to significant pleural adhesions in the emphysematous lung, concurrent left upper lobectomy was abandoned due to risk of phrenic nerve injury. He was therefore referred for definitive radiotherapy. Unexpectedly, the radiotherapy simulation CT revealed a reduction in the size of the LUL primary from 17 to 9 mm (Figure 3). Repeat FDG-PET/CT showed a significant reduction in FDG avidity of the primary lesion and ipsilateral station 10 lymph node from SUV 9.7 to 1.5 and 4.4 to 3.2, respectively (Figure 4).
His case was re-discussed at the MDT. A decision was made to pursue close surveillance with serial FDG-PET/CT imaging, with a plan for a fine needle aspirate of the left station 10 lymph node if persistent or increasing in avidity and/or size. Follow-up imaging confirmed persistent reduction in size of the lesion on CT to 9 mm with a corresponding SUV of 1.3. The ipsilateral station 10 lymph node now shows physiological avidity.
There are no readily apparent confounding factors for lesion resolution. The patient denies additional medications or supplements with immunomodulatory effects. The biopsy and CABG were uncomplicated with no wound infections or concurrent illness. As of the last follow-up in November 2022, over two years since diagnosis, there was no radiological evidence of recurrence.
Discussion
Spontaneous regression of cancer has been observed for centuries but gained its first scientific explanation by Everson and Cole in 1956, who defined it as “the partial or complete disappearance of a malignant tumor in the absence of all treatment, or in the presence of therapy which is considered inadequate to exert a significant influence on neoplastic disease” [6]. They theorized a range of factors such as infection, allergy or endocrine influences as potential explanations of the phenomenon [4],[6]. Notably absent from their discussion was the role of endogenous immune surveillance.
Cellular immunology and the concept of the body’s “immune system” first arose in the 1960s [7]. It is now recognized as playing an integral role in preventing cancer with evasion of the immune system one of cancer’s hallmarks [8]. Consequently, the immune system is crucial in SR and immune sensitizing events such as trauma, biopsy, and infection are often noted preceding tumor regression. Infections have long been recognized as immunomodulatory and have been used therapeutically to this effect. One of the most successful uses of immunotherapy is with intravesical Bacillus–Calmette–Guerin, an attenuated strain of mycobacterium bovis, for non-muscle invasive bladder cancer [9]. In our case, there was no clear coincident infection. Interestingly, in SR of lung cancer, biopsies and operative trauma are more commonly observed as possibly related events, rather than infection [4].
There are several mechanisms by which a biopsy may contribute to SR. Disturbance of the tumor microenvironment with passes of a core biopsy, could well be consequential due to the complex array of signaling interactions between the tumor microenvironment and malignant cells, which is increasingly recognized as an important factor in tumorigenesis [7],[8]. Alternatively, the biopsy may induce the abscopal effect, whereby local cellular stress or injury promotes liberation of “neoantigens” promoting a tumor-specific immune response and subsequent systemic effect [10].
PD-L1 prevents autoimmunity in physiological conditions, but is often exploited by cancers to evade immune recognition [11]. In NSCLC, high PD-L1 expression is associated with worse survival, but is also predictive of response to immunotherapy [12],[13]. Interestingly in our case, the tumor had a high PD-L1 expression of 65%. To our knowledge there have been no in-depth studies looking at the histopathology of lung cancers that undergo SR and whether certain biological markers contribute. Another case report of spontaneous regression in a locally advanced poorly differentiated NSCLC revealed a PD-L1 expression of 99% on retrospective analysis [14]. Further molecular characterization of cancers that undergo SR could potentially identify markers that are predictive and help unravel the biological mechanisms underpinning its occurrence.
Another question to be answered is the durability of SR. While the definition encapsulates both partial and sustained regression, it is unclear the proportion of each and how many cases result in “cure.” The literature, largely in the form of case reports or series, is typically limited in the length of follow-up. In the cases of SR in lung cancer compiled by Zhang et al., the majority had a follow-up period of two years or less [5]. The factors that differentiate cases of partial and sustained regression may be insightful with clonogenic populations that differ in key ways from the original tumor cell population seen in relapses.
Conclusion
Spontaneous regression of cancer is a misnomer as it belies specific mechanisms underlying the phenomenon. Idiopathic regression of cancer may be a more appropriate term as it signals that while unknown, there is an underlying etiology nonetheless. As our knowledge of the complex interplay between cancer cells, the tumor microenvironment and the immune system expands, a greater understanding of the molecular characteristics of cancers that undergo SR, and its triggers, may emerge. This may lead to improvement in the use of immunotherapeutics and the development of novel treatment targets.
REFERENCES
1.
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71(3):209–49. [CrossRef]
[Pubmed]

2.
Australia C. Lung cancer in Australia statistics. Cancer Australia. 2019. [Available at: https://www.canceraustralia.gov.au/cancer-types/lung-cancer/statistics]

3.
Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32(4):669–92. [CrossRef]
[Pubmed]

4.
Radha G, Lopus M. The spontaneous remission of cancer: Current insights and therapeutic significance. Transl Oncol 2021;14(9):101166. [CrossRef]
[Pubmed]

5.
Zhang J, Wang H, Li C, Qian H. Chance to rein in a cancer—Spontaneous regression of lung carcinoma (1988–2018): A 30-year perspective. Int J Clin Exp Pathol 2020;13(5):1190–6.
[Pubmed]

6.
Cole WH, Everson TC. Spontaneous regression of cancer: Preliminary report. Ann Surg 1956;144(3):366–83. [CrossRef]
[Pubmed]

7.
Moulin AM. The immune system: A key concept for the history of immunology. Hist Philos Life Sci 1989;11(2):221–36.
[Pubmed]

8.
Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144(5):646–74. [CrossRef]
[Pubmed]

9.
Gandhi NM, Morales A, Lamm DL. Bacillus Calmette-Guérin immunotherapy for genitourinary cancer. BJU Int 2013;112(3):288–97. [CrossRef]
[Pubmed]

10.
Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018;18(5):313–22. [CrossRef]
[Pubmed]

11.
Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. Pd-L1. J Clin Pathol 2018;71(3):189–94. [CrossRef]
[Pubmed]

12.
Li H, Xu Y, Wan B, et al. The clinicopathological and prognostic significance of PD-L1 expression assessed by immunohistochemistry in lung cancer: A meta-analysis of 50 studies with 11,383 patients. Transl Lung Cancer Res 2019;8(4):429–49. [CrossRef]
[Pubmed]

13.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372(21):2018–28. [CrossRef]
[Pubmed]

14.
Yoon HY, Park HS, Cho MS, Shim SS, Kim Y, Lee JH. Spontaneous remission of advanced progressive poorly differentiated non-small cell lung cancer: A case report and review of literature. BMC Pulm Med 2019;19(1):210. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Kelleher Retchford - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Catherine Bettington - Conception of the work, Design of the work, Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
St. John Newman - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2023 Kelleher Retchford et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.