|
Case Report
A case of thyroid sclerosing mucoepidermoid carcinoma with eosinophilia indicates interleukin-5 production in a man with a history of Graves’ disease
1 Department of Diagnostic Pathology, National Hospital Organization, Fukuyama Medical Center, Fukuyama, Japan
2 Department of Otolaryngology-Head and Neck Surgery, National Hospital Organization, Fukuyama Medical Center, Fukuyama, Japan
3 Department of Diagnostic Pathology, Okayama University Hospital, Japan
4 Department of Pathology and Translational Research, Gifu University Graduate School of Medicine, Gifu, Japan
Address correspondence to:
Hiroshi Sonobe
Department of Diagnostic Pathology, National Hospital Organization, Fukuyama Medical Center, Fukuyama,
Japan
Message to Corresponding Author
Article ID: 100114Z10HS2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Sonobe H, Omote R, Fukushima K, Yanai H, Niwa R, Saigo C. A case of thyroid sclerosing mucoepidermoid carcinoma with eosinophilia indicates interleukin-5 production in a man with a history of Graves’ disease. J Case Rep Images Oncology 2022;8(2):37–44.ABSTRACT
Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) is a rare tumor that typically affects women with Hashimoto’s thyroiditis. The present case was a man in his late 50s who was diagnosed with Graves’ disease at the age of 10 and was given antithyroid hormone for five years. The computed tomography scan revealed a nodular lesion in the right lobe, and the lesion was cytologically suspected as papillary carcinoma. No lymph node metastases or distant metastases were found. Before total thyroidectomy, high serum anti-thyroid peroxidase (TPO) and antithyroglobulin (TG) antibody titers, with no eosinophilia were detected. In a few small areas of the tumor center, small tumor cell foci with mild to moderate atypia, displaying mucous glandular cell and squamous cell differentiation, were found. The tumor was completely replaced by prominent sclerosing fibrosis, which was accompanied by tumor cell infiltration. The tumor had invaded the adjacent parenchyma and perithyroidal fatty tissue. In addition to lymphocytes and plasma cells, a large number of eosinophils were observed within the tumor. Immunohistochemically, tumor cells were strongly positive for p63, 34βE12, and TTF-1, but weakly for PAX8. Using fluorescence in situ hybridization (FISH), no MAML2 translocation was detected. Taken together with these findings, the present tumor was diagnosed as primary thyroid sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE). This case is the first to report thyroid SMECE associated with Graves’ disease. IL-5 immunostaining was performed to identify eosinophilia within the present tumor. As a result, the tumor cells were found to be positive for IL-5. The present tumor is also the first to indicate IL-5 production of SMECE.
Keywords: Interleukin-5 production, Graves’ disease, Male, Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia
Introduction
To date, a considerable number of mucoepidermoid carcinomas (MECs) have been reported. Most of them develop in the salivary glands [1],[2],[3], but also occur in the lungs [4], esophagus [5], cervix [6], thyroid [7],[8], and lacrimal [9]. Since the first report by Chan et al. in 1991 [10], more than 70 cases of sclerosing thyroid mucoepidermoid carcinoma (SMECE) with eosinophilia have been reported [11],[12],[13],[14],[15], with the exception of esophagus [16] and salivary gland [17]. Thyroid SMECE usually occurs in adult women with Hashimoto’s thyroiditis [10],[15], but no SMECE associated with thyroid disease other than Hashimoto’s thyroiditis is found as far as we know. Furthermore, eosinophilia within tumors is a typical histology for SMECE, but its histogenesis is still not understood. We report a recently experienced case of thyroid SMECE developing in a man in his late 50s who was diagnosed with Graves’ disease at age 10 years. In addition, we also report here that immunostaining with an anti-IL-5 antibody for clarifying the mechanism of eosinophilia indicated IL-5 production by the tumor cells, suggesting cause of eosinophilia.
Case Report
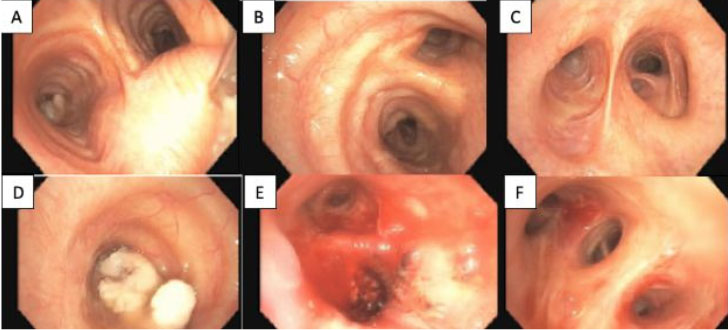
The present case was a man in his late 50s who had been skinny and tall since childhood with increasing appetite and exophthalmos. He was diagnosed with Graves’ disease after a laboratory analysis revealed a high titer of thyroid hormone, and was given antithyroid hormone for five years. A family doctor recently noticed a swelling in the right neck while the patient was being treated for hypertension and hyperlipidemia. A computed tomography (CT) scan performed at our hospital indicated a nodule developing from the right lobe to the thyroid isthmus (Figure 1). There were no detectable regional lymph node metastases or distant metastases. A cytological examination was performed, and papillary carcinoma was suspected. During the total thyroidectomy, no tumor infiltration into the trachea or perithyroidal muscles was detected. Before surgery, serum antithyroid peroxidase (TPO) antibody and anti-thyroglobulin (TG) antibody titers were high (381.0 and 42.4 IU/mL, respectively). Hematological data indicated mild anemia but not revealed eosinophilia (Table 1). At present, no signs of recurrence or metastasis are detected 14 months after surgery.
The cytological examination revealed epithelial cells with mild to moderate nuclear atypia in relatively flat and small clusters. There were also nuclear grooves and intranuclear pseudo-inclusion bodies were also observed (Figure 2A), with no inflammatory cells in the background. Since the cell components were sparse, the cytopathology suggested papillary carcinoma. The immunocytochemistry using the cell transfer method revealed that the cell clusters were strongly positive for 34βE12 (Figure 2B).
Macroscopically, the tumor was 3 × 2.5 cm in size, yellowish-white, solid hard with no capsule, and had infiltrated into the adjacent parenchyma (Figure 3A). Histologically, the tumor was completely replaced by prominent sclerosing fibrosis, which was accompanied by tumor cell infiltration (Figure 3B), and the tumor invaded the adjacent parenchyma and perithyroidal fatty tissues. In a few small areas of the tumor center, neoplastic epithelial components with moderate atypia displayed a variety of features, including irregular glandular and follicular patterns (Figure 3C), dilated glandular patterns with mucus retention (Figure 3D), sheet-like patterns of squamoid epithelia (Figure 3E), and a variable mixture in various proportions with mucinous glandular and squamous differentiation (Figure 4A). In addition, a large number of eosinophils as well as a considerable number of lymphocytes and plasma cells were observed within the lesion (Figure 4B). No calcified deposits or psammoma bodies were found. Immunohistochemically, tumor cells were strongly positive for p63 (Figure 4C), 34βE12, and thyroid transcription factor-1 (TTF-1), weakly for paired-box gene 8 (PAX8), and also positive for IL-5 (Figure 4D). The spindle-shaped cells in sclerosing fibrosis were negative for β catenin. There were only a few immunoglobulin G4 (IgG4)-positive plasma cells. Furthermore, the MAML2 translocation in tumor cells was examined by split fluorescence in situ hybridization (FISH) technique using MAML2(11q21) break apart probe which composed of RH92074 labeled red and AFM303TEF1 labeled red, and it was not detected. Based on the aforementioned findings, the present thyroid tumor was diagnosed as SMECE, indicating tumor cell production of IL-5 by tumor cells (Figure 5).
Discussion
In 1991, Chan et al. first reported eight cases of thyroid SMECE as a variant of thyroid MEC [10]. According to the descriptions, all eight patients were females with a history of Hashimoto’s thyroiditis, and the disease was clinically low malignant. Histologically, this tumor is characterized by tumor cell differentiation into glandular and squamous epithelia as well as prominent sclerosing fibrosis and eosinophilia within the lesion. The tumor origin is thought to be metaplasia of the follicular epithelia that occurs during the course of Hashimoto’s thyroiditis. On the other hand, Hunt et al. suggested that the SMECE originated from the ultimobranchial body (UBB), as both the tumor cells and the remnants of UBB/solid cell nests were positive for p63 [11]. Because of the tumor’s p63 positivity and the absence of a history of Hashimoto’s thyroiditis, the notion of UBB genesis appears plausible in this case.
Thyroid SMECE, as previously mentioned, typically develops in women with Hashimoto’s thyroiditis and is histologically characterized by prominent sclerosing fibrosis and eosinophilia in the lesion [8],[10],[12]. In contrast, thyroid MEC does not necessarily have Hashimoto’s thyroiditis as a background, and neither sclerosing fibrosis nor eosinophilia is present [7],[8]. Furthermore, MAML2 translocation is frequently detected in MEC [3]. However, there is no MAML2 translocation or specific genetic aberration in thyroid SMECE [13],[14]. Therefore, MEC and SMECE are distinct entities and are classified as such in the WHO classification (2017, 4th edition) [18]. Because of the extensive sclerosing fibrosis, thyroid SMECE has differential diagnoses of diffuse sclerosing papillary thyroid carcinoma (DS-PTC) [19],[20] and papillary thyroid carcinoma with fibromatosis-like stroma (PTC-FMS) [21],[22]. Diffuse sclerosing papillary thyroid carcinoma is characterized by prominent calcified deposits or numerous psammoma bodies [19]. The fibroma-like stroma in PTC-FMS is positive for β-catenin, indicating β-catenin gene aberration [21],[22]. Since β-catenin gene aberration is frequent in soft tissue fibromatosis, PTC-FMS is most likely regarded as a duplication of thyroid papillary carcinoma and fibromatosis [22]. The present tumor had prominent sclerosing fibrosis and eosinophilia. However, there was no papillary carcinomatous component or psammoma body, and spindle cells in the sclerosing fibrosis were negative for β-catenin. Furthermore, no MAML2 translocation could be detected (Table 2).
Regarding the clinicopathological features of thyroid SMECE, it was initially regarded as a low-grade malignancy [10], but reports contradicting this emerged [23],[24]. According to a recent analysis of 61 cases of thyroid SMECE [15], the tumor usually manifests as a painless, hard lesion in middle-aged to elderly women with Hashimoto’s disease, and it is most likely to occur in the right lobe. The tumor may have complications such as papillary carcinoma, lymph node metastasis, and distant metastasis when it first develops. Furthermore, recurrence, lymph node metastasis, and distant metastases after surgery, as well as death from the disease have been reported. Immunohistochemically, thyroid SMECE is strongly positive for p63 and mucicarmine, as well as for p40 and 34βE12, but not always for TTF1. The positivity rates of PAX8 and TG, which are markers of follicular epithelia, are low. As previously mentioned, the present case of thyroid SMECE was pathologically typical but clinically unusual. The case was a man with a history of Graves’ disease. To the best of our knowledge, no reports of thyroid SMECE associated with Graves’ disease have been published.
Recently, there have been reports referring to the dedifferentiation of thyroid SMECE. Mao et al. [25] reported a case with SMECE and undifferentiated carcinoma coexisting in one thyroid gland nodule, and the patient had local recurrence and distant metastases within a short period of 1 year and 3 months after surgery. Furthermore, Agaimy et al. [26] encountered an unusual case in which nuclear protein in testis midline carcinoma (NUT carcinoma), a high-grade malignancy, developed six months after thyroid SMECE surgery. Since the NUT carcinoma developed promptly after surgery, the authors assumed that it was derived from SMECE rather than a dual malignancy that developed at distinct times. These data suggest the dedifferentiation of SMECE, and the possibility of this phenomenon should be considered in cases of thyroid SMECE.
To date, there have not been many reports with thorough cytological findings in thyroid SMECE. Hirokawa et al. [14] found mucoepidermoid carcinoma cells with eosinophils in one of three cases. However, the definitive cytological diagnosis for thyroid SMECE is difficult, even though it may lead to a diagnosis of malignancy or suspicion [27],[28],[29]. Because intracytoplasmic pseudo-inclusion bodies were observed in mildly atypical epithelial cells, papillary carcinoma was suspected in the present case. However, immunostaining using the cell transfer method revealed a positive reactivity of tumor cells for 34βE12. As a result, the emerging cells, including an intracytoplasmic pseudo-inclusion body in cytology, were proven to be derived from the present SMECE. Therefore, we reexamined the present SMECE tissue but found no evidence of an intracytoplasmic inclusion body. The reason for the discrepancy between cytological and histological findings in the present tumor remains unclear.
Unlike MEC, SMECE displays the characteristic histological features of extensive sclerosing fibrosis and eosinophilia in the lesion, but its histogenesis has received little attention. Concerning the sclerosing fibrosis, the possibility of IgG4-related diseases was explored, because a number of IgG4 positive plasma cells appeared within the lesion in some cases [14],[17]. However, storiform fibrosis and obstructive phlebitis, which are specific to IgG4-related diseases, are not detected [30]. In another case, no significant IgG4 positive plasma cells were detected [13], and a sclerosing variant of MEC with the MALA2 translocation was reported [31]. Therefore, the mechanism of extensive fibrosis in SMECE is not fully understood. By the way, the present SMECE included only a small number of IgG4 positive plasma cells with no storiform fibrosis or obstructive phlebitis.
Interleukin-5 plays a central role in eosinophil production (eosinophilia), activation, and recruitment [32]. However, reports on IL-5 production in tumor cells are sparse, including lung non-small cell carcinoma [33], hepatocellular carcinoma [34], metastatic carcinoma [35], and mesothelioma [36]. In thyroid SMECE, not only primary, metastatic and recurrent tumors with eosinophilia are reported, but also pleural metastases with a large number of eosinophils in the effusion [27],[37]. Each tumor component in ordinary MEC or SMECE is morphologically identical but differs in the gene aspect. Therefore, we speculate that eosinophilia in SMECE is caused by IL-5 production by tumor cells and used immunostaining to identify the localization of IL-5 in the present SMECE. As a result, the tumor cells showed a positive reactivity to IL-5. Therefore, it is plausible to conclude that tumor cell production of IL-5 caused marked eosinophilia within the tumor. The present case is the first to indicate that tumor cells can produce IL-5 in SMECE.
Conclusion
We encountered a case of thyroid SMECE in a man with a history of Graves’ disease. To examine the histogenesis of eosinophilia within SMECE, we performed IL-5 immunostaining. As a result, tumor cells were positive for it, and this case is the first indication that tumor cells should produce IL-5 on SMECE. On the other hand, since some SMECEs have a highly malignant course, in order to save SMECE patients, we should recognize that it occurs not only in women but also in men and that it develops with thyroid diseases other than Hashimoto’s disease, and early diagnosis and surgery should be important.
REFERENCES
1.
Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: A clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001;25(7):835–45. [CrossRef]
[Pubmed]

2.
Bai S, Clubwala R, Adler E, et al. Salivary mucoepidermoid carcinoma: A multi-institutional review of 76 patients. Head Neck Pathol 2013;7(2):105–12. [CrossRef]
[Pubmed]

3.
Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol 2010;34(8):1106–21. [CrossRef]
[Pubmed]

4.
Huo Z, Wu H, Li J, et al. Primary pulmonary mucoepidermoid carcinoma: Histopathological and moleculargenetic studies of 26 cases. PLoS One 2015;10(11):e0143169. [CrossRef]
[Pubmed]

5.
Sasajima K, Watanabe M, Takubo K, Takai A, Yamashita K, Onda M. Mucoepidermoid carcinoma of the esophagus: Report of two cases and review of the literature. Endoscopy 1990;22(3):140–3. [CrossRef]
[Pubmed]

6.
Lennerz JKM, Perry A, Mills JC, Huettner PC, Pfeifer JD. Mucoepidermoid carcinoma of the cervix: Another tumor with the t(11;19)-associated CRTC1-MAML2 gene fusion. Am J Surg Pathol 2009;33(6):835–43. [CrossRef]
[Pubmed]

7.
Farhat NA, Faquin WC, Sadow PM. Primary mucoepidermoid carcinoma of the thyroid gland: A report of three cases and review of the literature. Endocr Pathol 2013;24(4):229–33. [CrossRef]
[Pubmed]

8.
Baloch ZW, Solomon AC, LiVolsi VA. Primary mucoepidermoid carcinoma and sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: A report of nine cases. Mod Pathol 2000;13(7):802–7. [CrossRef]
[Pubmed]

9.
Von Holstein SL, Fehr A, Heegaard S, Therkildsen MH, Stenman G. CRTC1-MAML2 gene fusion in mucoepidermoid carcinoma of the lacrimal gland. Oncol Rep 2012;27(5):1413–6. [CrossRef]
[Pubmed]

10.
Chan JK, Albores-Saavedra J, Battifora H, Carcangiu ML, Rosai J. Sclerosing mucoepidermoid thyroid carcinoma with eosinophilia. A distinctive low-grade malignancy arising from the metaplastic follicles of Hashimoto’s thyroiditis. Am J Surg Pathol 1991;15(5):438–48.
[Pubmed]

11.
Hunt JL, LiVolsi VA, Barnes EL. P63 expression in sclerosing mucoepidermoid carcinomas with eosinophilia arising in the thyroid. Mod Pathol 2004;17(5):526–9. [CrossRef]
[Pubmed]

12.
Shehadeh NJ, Vernick J, Lonardo F, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: A case report and review of the literature. Am J Otolaryngol 2004;25(1):48–53. [CrossRef]
[Pubmed]

13.
Shah AA, La Fortune K, Miller C, et al. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: A clinicopathologic and molecular analysis of a distinct entity. Mod Pathol 2017;30(3):329–39. [CrossRef]
[Pubmed]

14.
Hirokawa M, Takada N, Abe H, et al. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia distinct from the salivary type. Endocr J 2018;65(4):427–36. [CrossRef]
[Pubmed]

15.
Sukumar JS, Sukumar S, Purohit D, et al. Activating BRAF mutation in sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: Two case reports and review of the literature. J Med Case Rep 2019;13(1):385. [CrossRef]
[Pubmed]

16.
Kinoo SM, Maharaj K, Singh B, Govender M, Ramdial PK. Primary esophageal sclerosing mucoepidermoid carcinoma with “tissue eosinophilia”. World J Gastroenterol 2014;20(22):7055–60. [CrossRef]
[Pubmed]

17.
Tasaki T, Matsuyama A, Tabata T, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary gland: Case report and review of the literature. Pathol Int 2013;63(2):125–31. [CrossRef]
[Pubmed]

18.
19.
Pillai S, Gopalan V, Smith RA, Lam AKY. Diffuse sclerosing variant of papillary thyroid carcinoma—An update of its clinicopathological features and molecular biology. Crit Rev Oncol Hematol 2015;94(1):64–73. [CrossRef]
[Pubmed]

20.
Cavaco D, Martins AF, Cabrera R, Vilar H, Leite V. Diffuse sclerosing variant of papillary thyroid carcinoma: Outcomes of 33 cases. Eur Thyroid J 2022;11(1):e210020. [CrossRef]
[Pubmed]

21.
Takada N, Hirokawa M, Ito M, et al. Papillary thyroid carcinoma with desmoid-type fibromatosis: A clinical, pathological, and immunohistochemical study of 14 cases. Endocr J 2017;64(10):1017–23. [CrossRef]
[Pubmed]

22.
Rebecchini C, Nobile A, Piana S, et al. Papillary thyroid carcinoma with nodular fasciitis-like stroma and β-catenin mutations should be renamed papillary thyroid carcinoma with desmoid-type fibromatosis. Mod Pathol 2017;30(2):236–45. [CrossRef]
[Pubmed]

23.
Sim SJ, Ro JY, Ordonez NG, Cleary KR, Ayala AG. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: Report of two patients, one with distant metastasis, and review of the literature. Hum Pathol 1997;28(9):1091–6. [CrossRef]
[Pubmed]

24.
Quiroga-Garza G, Lee JH, El-Naggar A, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: More aggressive than previously reported. Hum Pathol 2015;46(5):725–31. [CrossRef]
[Pubmed]

25.
Mao R, Shi L, Yan W, Li W, Li B, Li X. Anaplastic thyroid carcinoma combined with sclerosing mucoepidermoid carcinoma with eosinophilia: A case report. Medicine (Baltimore) 2020;99(42):e22783. [CrossRef]
[Pubmed]

26.
Agaimy A, Tögel L, Stoehr R, et al. NSD3-NUTM1-rearranged carcinoma of the median neck/thyroid bed developing after recent thyroidectomy for sclerosing mucoepidermoid carcinoma with eosinophilia: Report of an extraordinary case. Virchows Arch 2021;479(6):1095–9. [CrossRef]
[Pubmed]

27.
Geisinger KR, Steffee CH, McGee RS, Woodruff RD, Buss DH. The cytomorphologic features of sclerosing mucoepidermoid carcinoma of the thyroid gland with eosinophilia. Am J Clin Pathol 1998;109(3):294–301. [CrossRef]
[Pubmed]

28.
Ames E, Campbell MJ, Afify A, Krane JF, Huang EC. Sclerosing mucoepidermoid carcinoma with eosinophilia: Cytologic characterization of a rare distinct entity in the thyroid. Diagn Cytopathol 2018;46(7):632–5. [CrossRef]
[Pubmed]

29.
Nam JH, Han CW, Kim NI, Kim SS, Choi YD. The cytological features of sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: A case report. Cytopathology 2020;31(6):593–7. [CrossRef]
[Pubmed]

30.
Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet 2015;385(9976):1460–71. [CrossRef]
[Pubmed]

31.
Yabuki K, Matsuyama A, Shiba E, Nagatani G, Hisaoka M. Sclerosing mucoepidermoid carcinoma in the parotid gland with CRTC1-MAML2 fusion: A case report. Int J Surg Pathol 2018;26(3):250–5. [CrossRef]
[Pubmed]

32.
Dent LA, Strath M, Mellor AL. Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med 1990;172(5):1425–31. [CrossRef]
[Pubmed]

33.
Pandit R, Scholnik A, Wulfekuhler L, Dimitrov N. Non-small-cell lung cancer associated with excessive eosinophilia and secretion of interleukin-5 as a paraneoplastic syndrome. Am J Hematol 2007;82(3):234–7. [CrossRef]
[Pubmed]

34.
Balian A, Bonte E, Naveau S, et al. Intratumoral production of interleukin-5 leading to paraneoplastic peripheral eosinophilia in hepatocellular carcinoma. J Hepatol 2001;34(2):355–6. [CrossRef]
[Pubmed]

35.
Fridlender ZG, Simon HU, Shalit M. Metastatic carcinoma presenting with concomitant eosinophilia and thromboembolism. Am J Med Sci 2003;326(2):98–101. [CrossRef]
[Pubmed]

36.
Takeuchi E, Takahashi N, Morizumi S, et al. Interleukin-5-producing malignant pleural mesothelioma with eosinophilic pleural effusion. Thorac Cancer 2020;11(10):3043–6. [CrossRef]
[Pubmed]

37.
Kobayashi Y, Satoh K, Aizawa T, Urano M, Kuroda M, Mizutani H. Local recurrence of sclerosing mucoepidermoid carcinoma with eosinophilia in the upper lip: A case report. J Med Case Rep 2015;9:41. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
We thank all the staffs of the Department of Diagnostic Pathology, NHO Fukuyama Medical Center for various support for this case.
Author ContributionsHiroshi Sonobe - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rika Omote - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Kei Fukushima - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hiroyuki Yanai - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Riko Niwa - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Chiemi Saigo - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Hiroshi Sonobe et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.