|
Case Report
Globe-salvaging treatment of amelanotic uveal melanoma with posterior extraocular extension
1 Division of Ocular Oncology, Edward S. Harkness Eye Institute, Columbia University Irving Medical Center, New York, NY, USA
2 Department of Pathology, Columbia University Irving Medical Center, New York, NY, USA
3 Department of Ophthalmology, Albany Medical Center, Albany, NY, USA
Address correspondence to:
Arpita S Maniar
MD, Department of Ophthalmology, Edward S. Harkness Eye Institute, 635 West 165th Street, New York, NY 10032,
USA
Message to Corresponding Author
Article ID: 100091Z10AM2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Maniar AS, Niedt G, Langevin ST, Marr BP. Globe-salvaging treatment of amelanotic uveal melanoma with posterior extraocular extension. J Case Rep Images Oncology 2021;7:100091Z10AM2021.ABSTRACT
Introduction: Extraocular extension (EOE) of uveal melanoma is rare and generally associated with poor systemic prognosis.
Case Report: We report a patient with amelanotic uveal melanoma with an extraocular extension managed with combination of surgical resection of the posterior extraocular extension along with transpupillary thermotherapy (TTT) to the choroidal lesion, thereby salvaging the globe. To the best of our knowledge, we are the first case to report such combination treatment for amelanotic uveal melanoma with posterior extension without evidence of orbital recurrence or systemic metastasis over a follow-up period of thirteen years.
Conclusion: Careful resection of the extraocular tumor component along with aggressive treatment of the primary intraocular tumor may achieve globe salvage in carefully selected cases.
Keywords: Extra ocular extension, Transpupillary thermotherapy, Uveal melanoma
Introduction
Approximately 85% of ocular melanomas arise from the uveal tract (including the iris, ciliary body, and choroid), and the remainder arise in the conjunctiva or (rarely) the orbit. Extraocular extension (EOE) of uveal melanoma is rare and occurs in 3–15% patients [1],[2],[3]. Historically, it has been associated with poor survival prognosis mandating aggressive management. With the advances in radiotherapy, the treatment paradigm for selected cases of extraocular uveal melanoma extension has evolved from exenteration or enucleation, to globe-sparing procedures. Factors involving the primary uveal tumor such as large intraocular tumor size, diffuse tumor, optic nerve invasion, epithelioid or mixed cell type, monosomy 3 and class II genetic mutations predict the development of metastasis [1],[4]. The survival in these patients is influenced by characteristics of the primary tumor itself more than the choice of treatment modality.
Currently, small uveal melanomas (tumor thickness ≤3 mm) are primarily treated with lasers such as transpupillary therapy and photodynamic therapy (PDT) in order to better preserve vision [5],[6]. In this report, we describe a patient with amelanotic uveal melanoma with a large posterior EOE managed by complete surgical resection of the extraocular component followed by transpupillary thermotherapy (TTT) to the residual intraocular choroidal lesion, with no evidence of orbital or systemic recurrence at 13 years follow-up. Health Insurance Portability and Accountability Act (HIPAA) compliance was ensured and we adhered to the declaration of Tenets of Helsinki.
Case Report
A 69-year-old Caucasian woman was referred to the oncology clinic for evaluation of an amelanotic choroidal lesion in the right eye. She had a history of bilateral mastectomy and lymph node dissection for breast cancer at the age of 40. She underwent mitral valve replacement, due to mitral valve disease, as a result of rheumatic fever in childhood. Cardiac history was also significant for atrial fibrillation with coronary artery disease. Her family history was significant for colon cancer in mother and breast cancer in sister.
Five years earlier, she had consulted her primary ophthalmologist for diminished vision in the same eye and was found to have an amelanotic choroidal lesion overlying the posterior pole. She was then referred to an ocular oncologist where the lesion was presumed to be a choroidal schwannoma versus amelanotic choroidal melanoma and observed. The lesion was monitored closely with minimal increase in the size, albeit slow visual deterioration, and increasing proptosis in the right eye over the years.
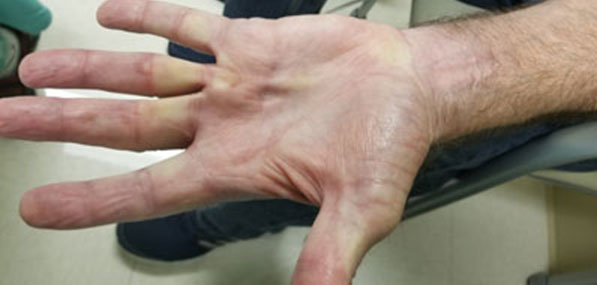
On examination in our clinic, she had right eye axial proptosis with full extraocular movements (Figure 1). Her best corrected visual acuity (BCVA) was 20/70 in the right eye (OD) and 20/25 in the left eye (OS). Anterior chamber showed no signs of inflammation. Right eye was pseudophakic whereas left eye showed early cataractous changes. Intraocular pressures were normal in both eyes. Fundus examination (OD) was significant for an amelanotic choroidal mass measuring 9 mm × 6 mm × 3 mm at the posterior pole, with choroidal folds (Figure 2A). There was no associated subretinal fluid or overlying orange pigment.
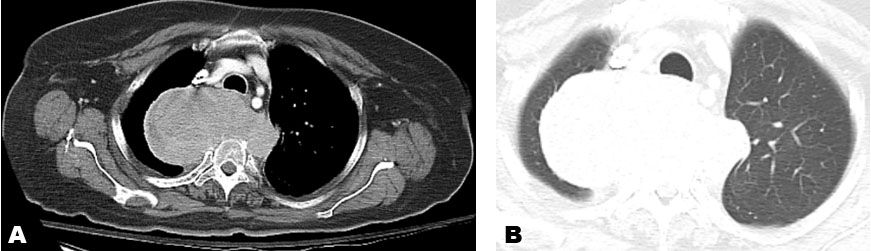
B-scan ultrasonography revealed a dome-shaped choroidal lesion with a rather disproportionately large contiguous iso-echoic dome-shaped extrascleral extension measuring 22.5 mm wide and 13.5 mm in height (Figure 2B). Magnetic resonance imaging (MRI) of the orbits confirmed an intraocular mass at the macula in the right eye along with a retrobulbar contiguous lesion causing proptosis. Clinical differential diagnosis at this point was choroidal schwannoma versus an amelanotic choroidal melanoma with extraocular extension. Systemic evaluation in the form of abdominal and chest imaging was negative for any lesions.
The different treatment options considered at this point were extended enucleation, exenteration versus excision of the extraocular tumor component followed by local treatment of primary tumor. As the lesion was slow-growing, we decided to first perform a biopsy of the orbital component of the tumor to ascertain the diagnosis. A transcutaneous orbitotomy was performed to access the extraocular component of the tumor. Intra-operatively, it appeared to be a soft encapsulated, amelanotic yellow white mass anatomically overlying and adhering to the sclera under the intraocular tumor. While dissecting the tumor, a clear tissue plane could be delineated between the tumor and the sclera, and the lesion was excised in toto. The scleral base of the tumor was then cauterized using bipolar cautery.
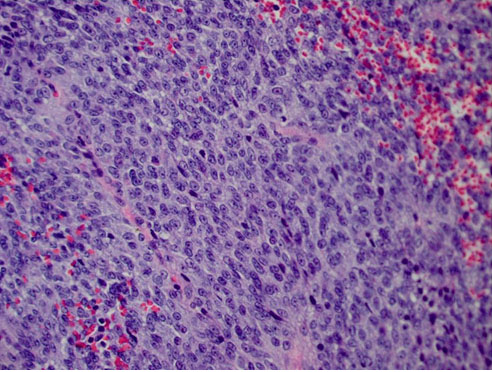
Histopathology revealed a mixed spindle and epithelioid cell malignant neoplasm (Figure 3) with positive immunostaining for S-100, melan-A, HMB45, and A103. Immunohistochemistry (IHC) was negative for smooth muscle markers such as desmin and glial fibrillary acidic protein (GFAP) which is frequently expressed in schwannoma [7],[8]. Immunohistochemistry was also negative cytokeratin markers AE1/AE3. Thus, the atypical clinical course, histopathology, and immunohistochemistry supported the diagnosis of amelanotic choroidal melanoma with extraocular extension. Fluorescence in situ hybridization (FISH) did not detect monosomy 3.
The different treatment options at this point were exenteration or enucleation versus local treatment of the intraocular component using unshielded plaque brachytherapy or transpupillary thermotherapy (TTT); based on patient preferences, the latter was chosen. TTT was delivered using an 810 nm diode laser via slit lamp biomicroscopy. A high power of 500–800 mW was used with a spot size of 3 mm and duration of 1 minute. Thermotherapy was delivered to the entire tumor along with a margin of the adjacent normal choroid in an overlapping manner. Three such cycles were performed two month apart. Post-treatment, the tumor showed good local regression (Figure 2C). After completion of the treatment, vision in the right eye was finger counting close to face. B scan ultrasonography was performed at each follow-up visit which confirmed intraocular tumor regression with negative orbital component (Figure 2D). The findings corroborated with annual MRI orbits which showed no recurrence of the orbital component. The patient was annually screened for metastasis with abdominal and chest imaging. Thirteen years post-treatment, there was no evidence of local recurrence or systemic metastasis and she maintained a vision of hand movement (HM) in the involved eye.
Discussion
The incidence of extraocular extension (EOE) in uveal melanoma is 3–15% and up to 5.5% patients [9] present with EOE at the first visit. Conflicting theories exist in association with the occurrence of extraocular extension and associated treatment modalities as well as survival. Here we describe a unique report of effective, long-term control of uveal melanoma with posterior EOE with globe-salvage wherein the extraocular component was surgically resected and the primary tumor was treated with transpupillary thermotherapy.
Extrascleral tumor extension may occur via aqueous channels, transsclerally via ciliary arteries, nerves or vortex veins or optic nerve; rarely a combination of above. Coupland et al. investigated routes of extrascleral tumor extension and did not find increased risk of metastasis with a particular route [10].
Five decades ago, Starr and Zimmerman (1962) found higher orbital recurrence and poor survival associated with extrascleral extension of uveal melanoma [2]. Shammas et al. (1977) suggested aggressive treatment with early exenteration in such patients [3]. Over the years, however, these beliefs have changed. Augsburger et al. (2000) and Gunduz et al. (2004) suggested that with similar primary tumor characteristics, there was no difference in survival prognosis for enucleation/exenteration versus globe sparing procedures [11],[12]. With recent advances in molecular diagnostics, gene expression profiling (GEP) is currently considered one of the most significant factors predicting tumor metastasis wherein Class II tumors are more likely to metastasize compared to Class I tumors [4],[13].
Whereas treatment of massive extraocular extension, non-encapsulated tumors and surgically transected tumors with exenteration is definitely agreed upon, extraocular extension with small extraocular component (≤3 mm tumor thickness) and moderate sized intraocular tumor (up to 5–6 mm) fall within a gray zone of enucleation versus globe preserving treatments. Gunduz et al. described successful use of plaque brachytherapy to treat extraocular extension with less than 3 mm thickness [12]. None of the patients in their series developed orbital recurrence and 18% patients died of systemic metastasis at three years. Seibel et al. studied the long-term outcomes of primary proton beam radiotherapy in the treatment of EOE [14]. They found no local recurrences with similar metastatic potential compared to cases without EOE. With increasing expertise in radiation delivery methods, the practice pattern has now shifted to globe preserving modalities. Besides radiotherapy, modalities like TTT and PDT are being used in the treatment of small uveal melanomas, typically with a height of ≤ 3 mm.
To the best of our knowledge, this is the first case in literature wherein an amelanotic intraocular component of uveal melanoma was successfully treated with transpupillary thermotherapy after resection of a significantly large posterior extraocular component. In our case, the decision to use TTT as an adjunct to surgical excision was based on intraocular tumor height of 3 mm, slow growing nature of the tumor located at the macula, operator experience as well as patient preference. Thirteen years following treatment and eighteen years from the detection of tumor, there is no evidence of local recurrence or systemic metastasis. In retrospect, gene expression profiling would have given us more insight into the case if it was available.
Conclusion
This case is a unique example that with careful resection and aggressive local treatment globe salvage may be obtained and be durable in eyes that have surprisingly large extraocular extension. Currently preoperative fine needle biopsy of the tumor with genetic analysis may help make treatment choices in these difficult cases, reserving globe salvage to genetically less aggressive tumors.
REFERENCES
1.
Affeldt JC, Minckler DS, Azen SP, Yeh L. Prognosis in uveal melanoma with extrascleral extension. Arch Ophthalmol 1980;98(11):1975–9. [CrossRef]
[Pubmed]

2.
3.
Shammas HF, Blodi FC. Orbital extension of choroidal and ciliary body melanomas. Arch Ophthalmol 1977;95(11):2002–5. [CrossRef]
[Pubmed]

4.
Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res 2004;64(20):7205–9. [CrossRef]
[Pubmed]

5.
Aaberg TM Jr, Bergstrom CS, Hickner ZJ, Lynn MJ. Long-term results of primary transpupillary thermal therapy for the treatment of choroidal malignant melanoma. Br J Ophthalmol 2008;92(6):741–6. [CrossRef]
[Pubmed]

6.
Donaldson MJ, Lim L, Harper CA, Mackenzie J, Campbell WG. Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin Exp Ophthalmol 2005;33(5):548–9. [CrossRef]
[Pubmed]

7.
8.
Udyaver S, Lim LAS, Milman T, Mashayekhi A, Shields JA, Shields CL. Intraocular schwannoma with extrascleral extension. Eur J Ophthalmol 2020;1120672120920211. [CrossRef]
[Pubmed]

9.
Bellmann C, Lumbroso-Le Rouic L, Levy C, et al. Uveal melanoma: Management and outcome of patients with extraocular spread. Br J Ophthalmol 2010;94(5):569–74. [CrossRef]
[Pubmed]

10.
Coupland SE, Campbell I, Damato B. Routes of extraocular extension of uveal melanoma: Risk factors and influence on survival probability. Ophthalmology 2008;115(10):1778–85. [CrossRef]
[Pubmed]

11.
Augsburger JJ, Schneider S, Narayana A, et al. Plaque radiotherapy for choroidal and ciliochoroidal melanomas with limited nodular extrascleral extension. Can J Ophthalmol 2004;39(4):380–7. [CrossRef]
[Pubmed]

12.
Gündüz K, Shields CL, Shields JA, Cater J, Brady L. Plaque radiotherapy for management of ciliary body and choroidal melanoma with extraocular extension. Am J Ophthalmol 2000;130(1):97–102. [CrossRef]
[Pubmed]

13.
Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: Prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012;119(8):1596–603. [CrossRef]
[Pubmed]

14.
Seibel I, Riechardt AI, Erb-Eigner K, et al. Proton beam irradiation: A safe procedure in postequatorial extraocular extension from uveal melanoma. Am J Ophthalmol 2018;191:49–53. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Arpita S Maniar - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
George Niedt - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Spencer T Langevin - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Brian P Marr - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Arpita S Maniar et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.