|
Case Series
Association of BRCAness with taxane resistance in triple-negative breast cancer: A study of 7 cases
1 MD, Lecturer, Department of Breast Oncology and Surgery, Tokyo Medical University, 6-7-1 Nishishinjuku, Shinjuku-ku, Tokyo, Japan
2 MD, Assistant Professor, Department of Breast Oncology and Surgery, Tokyo Medical University, 6-7-1 Nishishinjuku, Shinjuku-ku, Tokyo, Japan
3 MD, Associate Professor, Department of Anatomic Pathology, Tokyo Medical University, 6-7-1 Nishishinjuku, Shinjuku-ku, Tokyo, Japan
4 MD, Medical Director, Department of Breast Surgery, The Second Kawasaki Saiwai Clinic, 39-1 Miyako-cho, Saiwai-ku, Kawasaki-shi, Kanagawa, Japan
5 MD, Chief Professor, Department of Breast Oncology and Surgery, Tokyo Medical University, 6-7-1 Nishishinjuku, Shinjuku-ku, Tokyo, Japan
Address correspondence to:
Fuyo Kimura
Department of Breast Oncology and Surgery, Tokyo Medical University, 6-7-1 Nishishinjuku, Shinjuku-ku, Tokyo 160-0023,
Japan
Message to Corresponding Author
Article ID: 100089Z10FK2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Kimura F, Teraoka S, Sato E, Miyahara K, Nakamura Y, Ishikawa T. Association of BRCAness with taxane resistance in triple-negative breast cancer: A study of 7 cases. J Case Rep Images Oncology 2021;7:100089Z10FK2021.ABSTRACT
Introduction: Chemotherapy is the only systemic treatment option for triple-negative breast cancer (TNBC) patients, and is often used in combination with anthracycline and taxane. However, it was reported that many TNBCs show BRCA dysfunction, and are sensitive to DNA-damaging agents, but tend to be resistant to mitotic poisons, such as taxanes.
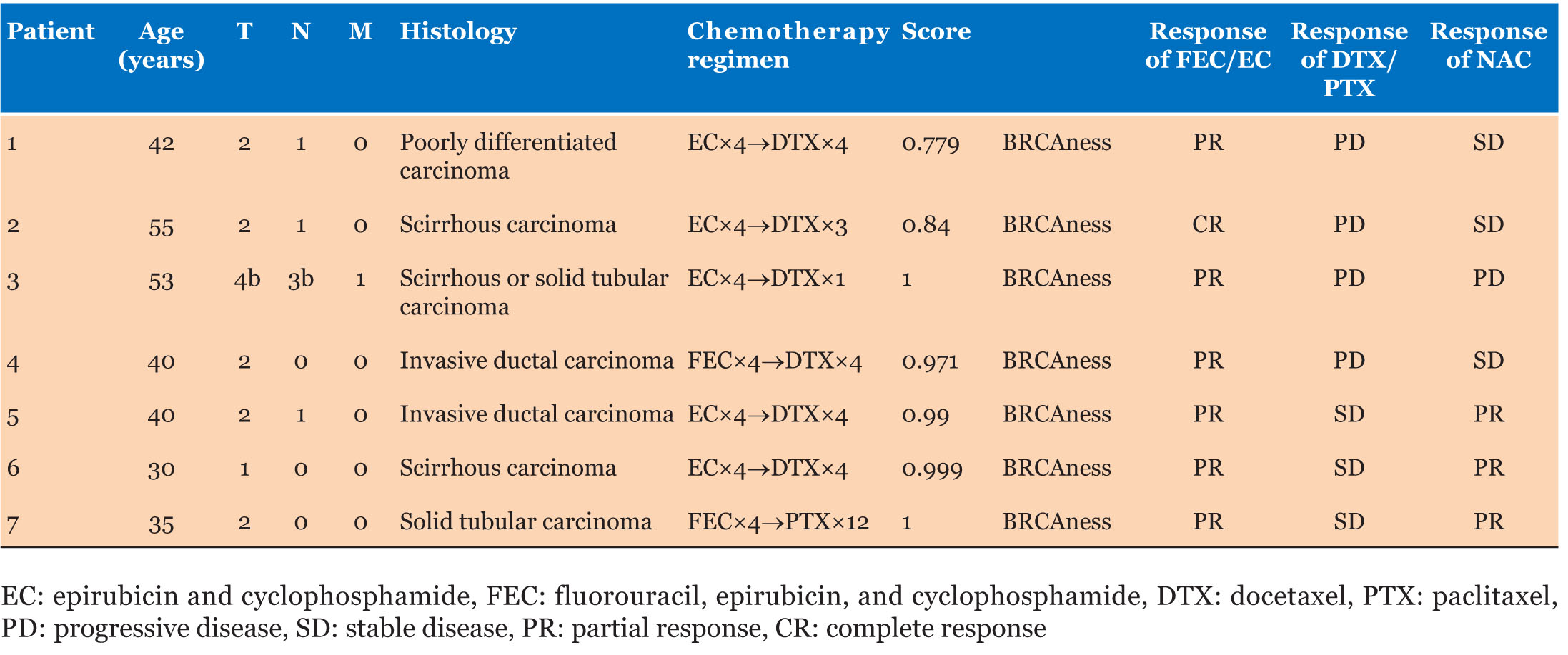
Case Series: Triple-negative breast cancer patients who underwent neoadjuvant chemotherapy with anthracycline followed by taxane were analyzed. Clinical responses to taxane were evaluated by the percentage of shrinkage in tumor size after anthracycline treatment. BRCAness was analyzed by mutiplex ligation-dependent probe amplification (MLPA) in core-needle biopsy specimens of 7 patients with progressive disease or stable disease after taxane treatment. BRCAness scores of the 7 patients were between 0.779 and 1.000 (0.939 ± 0.084), and all were categorized as having BRCAness.
Conclusion: Patients resistant to taxane were categorized as having BRCAness by MLPA. Mutiplex ligation-dependent probe amplification may hence be a promising method to identify TNBC patients with taxane resistance.
Keywords: BRCAness, Resistance, Taxane, Triple-negative breast cancer
Introduction
Breast cancer treatment is determined based on each patient’s state of the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor. Triple-negative breast cancer (TNBC) is defined as a subtype in which all of these receptors are negative, and comprises 10–20% of breast cancers. However, genetic expression profiles demonstrated that TNBC is a heterogeneous disease, with the following 6 subtypes: basal-like (BL)1, BL2, immunomodulatory, stem-cell like, luminal-androgen receptor (LAR), and mesenchymal-like [1],[2]. Sensitivities to anticancer agents have been reported to be different among the subtypes. In a previous study on neoadjuvant chemotherapy (NAC), the pathological complete response rate was 63% in patients with BL1 and BL2, whereas it was 31% and 14% in patients with mesenchymal-like and LAR, respectively [3].
BRCA1 is a gene that is associated with hereditary breast cancer. In mammary epithelial development, this mutation stops further differentiation, and hence this gene is involved in the occurrence of basal-like breast cancer [4]. Only 10–20% of patients are found to have a germline BRCA1 mutation [5]. However, BRCA1 dysfunction has been observed in 60–70% of TNBCs, and is referred to as BRCAness [5],[6].
Several clinical studies reported that BRCA1 mutation carriers have a lower response to taxane than non-mutation carriers [7],[8]. Furthermore, previous clinical studies of neoadjuvant and adjuvant chemotherapy suggested that BRCAness is also associated with sensitivity to DNA-damaging agents and resistance to mitotic poisons [9],[10],[11],[12]. Therefore, BRCAness may be useful for subdividing TNBCs for selecting the most effective chemotherapy regimens. However, both anthracycline and taxane are presently used for treating all TNBC patients. It would hence be beneficial to identify patients who are resistant to taxane, before starting treatment. Thus, we performed a pilot study to analyze the efficacies of taxane as NACs in treating TNBC, as well as the potential of clarifying BRCAness by multiple ligation-dependent probe amplification (MLPA), to identify taxane-resistant TNBC patients.
Case Series
Triple-negative breast cancer patients who underwent successive NAC treatment with anthracycline followed by taxane were analyzed. Patients underwent magnetic resonance imaging and/or ultrasound at 3 time points; i.e., before NAC, after anthracycline treatment, and after completing NAC.
Clinical responses to epirubicin and cyclophosphamide (EC)/fluorouracil, epirubicin, and cyclophosphamide (FEC), and docetaxel (DTX)/paclitaxel (PTX) were evaluated by measuring changes in tumor diameter after completing the anthracycline regimens and taxane regimens, and were calculated as a percentage. An increase in tumor diameter of 20% or more was considered to be progressive disease (PD), whereas an increase of less than 20% to a decrease of less than 30% was considered to be stable disease (SD). With regard to DTX/PTX, the effectiveness was evaluated by comparing the diameter of the tumor with that at the end of the EC/FEC treatment. Ten patients in this case control study were found to be resistant to taxane (PD or SD) from their medical records, and paraffin-embedded block samples of the core-needle biopsy at diagnosis were available for 7 out of the 10 patients. These were cut into thin sections (volume: 1.4×22×0.01×2 mm3), and DNA was extracted using QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). Results obtained by the MLPA method were scored based on prediction analysis for microarrays; a cutoff value of 0.5 or higher was taken to be BRCA1-like (equivalent to BRCAness). According to previous reports, the MLPA method was performed by Falco Biosystems Ltd. (Kyoto, Japan) using P376-B2 BRCA1ness probemix (MRC-Holland, Amsterdam, The Netherlands) [13]. Sample volumes sufficient for BRCAness analysis were available for 7 patients. BRCA-like scores of these patients were between 0.779 and 1.000 (0.939 ± 0.084), and all 7 patients were categorized as having BRCAness (Table 1). Changes in tumor diameter of the patients with BRCAness are shown in Figure 1.
Discussion
We found that all patients with PD or SD after taxane treatment had BRCAness, although they showed favorable responses to anthracycline treatment. These findings are consistent with our clinical experience that tumors sometimes shrink during anthracycline regimens and regrow during taxane regimens when performing NAC in TNBC patients. These findings imply that TNBC is generally more sensitive to anthracycline than taxane, or includes a subgroup that is resistant to taxane, and this may be associated with BRCA dysfunction in some TNBC patients. There are various strategies to identify the molecular features of BRCA dysfunction, including the sequencing of known homologous recombination (HR) genes, the promoter hypermethylation assay, identification of transcriptional metagene signatures, copy-number-based methods, functional assays of HR competence, and MLPA, which have all been developed to detect BRCA1/BRCA2 deficiency [13],[14],[15]. Among these, we focused on MLPA, because our previous randomized phase II clinical study demonstrated that BRCAness tumors are more sensitive to anthracycline than taxane [9].
In the present study, all 7 PD and SD patients during PTX/DTX treatment were categorized as having BRCAness. A previous study showed that 60–70% of patients have BRCAness among the general population of TNBC patients [6], which is clearly a high percentage. Based on the results of this pilot study and our previous study, we have started a prospective single-arm study of NAC by anthracycline regimens without taxane for patients with BRCAness. It is necessary to perform MLPA in all patients to clarify the association between BRCAness identified by MLPA and taxane resistance, as well as to compare BRCAness scores between patients with taxane resistance and those sensitive to chemotherapy. Some limitations of this study are that BRCAness scores were only measured in a small number of patients, and those chemoresistant patients and chemosensitive patients were not analyzed in this pilot study. Hence, we plan to perform further analyses of these points in a future study.
Conclusion
The results of our study suggest that some TNBCs are resistant to taxane, and the analysis of BRCAness by MLPA may be clinically useful for the identification of TNBC patients who are resistant to taxane, because this method is relatively simple and can be performed using paraffin-embedded tissue.
REFERENCES
1.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363(20):1938–48. [CrossRef]
[Pubmed]

2.
Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121(7):2750–67. [CrossRef]
[Pubmed]

3.
Lehmann BD, Bauer JA, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One 2016;16;11(6):e0157368. [CrossRef]
[Pubmed]

4.
Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist 2011;16 Suppl 1:61–70.
[Pubmed]

5.
Brianese RC, Nakamura KDM, de Almeida FGDSR, et al. BRCA1 deficiency is a recurrent event in early-onset triple-negative breast cancer: A comprehensive analysis of germline mutations and somatic promoter methylation. Breast Cancer Res Treat 2018;167(3):803–14.
[Pubmed]

6.
Lips EH, Mulder L, Oonk A, et al. Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer 2013;108(10):2172–7. [CrossRef]
[Pubmed]

7.
Byrski T, Gronwald J, Huzarski T, et al. Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat 2008;108(2):289–96. [CrossRef]
[Pubmed]

8.
Kriege M, Jager A, Hooning MJ, et al. The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer. 2012;118(4):899–907. [CrossRef]
[Pubmed]

9.
Ishikawa T, Narui K, Tanabe M, et al. BRCAness is beneficial for indicating triple negative breast cancer patients resistant to taxane. Eur J Surg Oncol 2016;42(7):999–1001. [CrossRef]
[Pubmed]

10.
Mori H, Kubo M, Nishimura R, et al. BRCAness as a biomarker for predicting prognosis and response to anthracycline-based adjuvant chemotherapy for patients with triple-negative breast cancer. PLoS One 2016;11(12):e0167016. [CrossRef]
[Pubmed]

11.
Liu L, Matsunaga Y, Tsurutani J, et al. BRCAness as a prognostic indicator in patients with early breast cancer. Sci Rep 2020;10(1):21173. [CrossRef]
[Pubmed]

12.
Severson TM, Peeters J, Majewski I, et al. BRCA1-like signature in triple negative breast cancer: Molecular and clinical characterization reveals subgroups with therapeutic potential. Mol Oncol 2015;9(8):1528–38. [CrossRef]
[Pubmed]

13.
Lips EH, Laddach N, Savola SP, et al. Quantitative copy number analysis by Multiplex Ligation-dependent Probe Amplification (MLPA) of BRCA1-associated breast cancer regions identifies BRCAness. Breast Cancer Res 2011;13(5):R107. [CrossRef]
[Pubmed]

14.
Staaf J, Glodzik D, Bosch A, et al. Whole-genome sequencing of triple-negative breast cancers in a population-based clinical study. Nat Med 2019;25(10):1526–33. [CrossRef]
[Pubmed]

15.
Glodzik D, Bosch A, Hartman J, et al. Comprehensive molecular comparison of BRCA1 hypermethylated and BRCA1 mutated triple negative breast cancers. Nat Commun 2020;11(1):3747. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
We thank the Department of International Medical Communications of Tokyo Medical University for reviewing and editing the manuscript.
Author ContributionsFuyo Kimura - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Saeko Teraoka - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Eiichi Sato - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Kana Miyahara - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yukiko Nakamura - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Takashi Ishikawa - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Fuyo Kimura et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.