|
Case Report
Volumetric modulated arc therapy (VMAT) craniospinal image-guided radiotherapy and chemotherapy for high-risk medulloblastoma in adults: A case report with analysis of the technique
1 Department of Radiotherapy/Oncology, University Hospital of Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece
Address correspondence to:
Michael I Koukourakis
MD, Department of Radiotherapy/Oncology, Democritus University of Thrace, Alexandroupolis 68100,
Greece
Message to Corresponding Author
Article ID: 100087Z10CN2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Nanos CA, Abatzoglou I, Koukourakis MI. Volumetric modulated arc therapy (VMAT) craniospinal image-guided radiotherapy and chemotherapy for high-risk medulloblastoma in adults: A case report with analysis of the technique. J Case Rep Images Oncology 2021;7:100087Z10CN2021.ABSTRACT
Introduction: Adult medulloblastoma is a rare disease. Due to the length of the body areas demanding irradiation, medulloblastoma is a challenging malignancy for the development of advanced radiotherapy techniques.
Case Report: We describe a volumetric modulated arc therapy (VMAT) technique applied under image-guided radiation therapy (IGRT) for craniospinal irradiation, offered to a patient with high risk medulloblastoma, together with cisplatin/temozolomide chemotherapy. Three areas of irradiation (cranial, thoracic, and lumbar) were defined, with shifting the gaps between them at a weekly basis (two shifts within the three weeks of therapy). The biological equivalent “normalized total dose” (NTD) to the craniospinal axis and the gaps between the three VMAT-treated areas were calculated for α/β-ratio of 2 Gy, and all the data from dose volume histograms (DVHs) are shown.
Conclusion: The technique is reliable delivering with safety and accuracy the desirable radiation dose, with biologically and oncologically acceptable dose-deviations within the field-gaps. The tolerance of the radiochemotherapy regimen was excellent.
Keywords: Craniospinal irradiation, Medulloblastoma, VMAT
Introduction
Medulloblastomas are the most common malignant brain tumors in children, with an incidence of six new cases per million population per year that drops to two new cases in the second decade of life [1]. This is, however, a rare disease in adults with a ten-fold decreased incidence compared to children [2]. Medulloblastomas are highly invasive embryonal neuroepithelial tumors arising in the fourth ventricle and disseminate throughout the subarachnoid system via the cerebrospinal fluid. Extraneural metastasis may also occur in less than 10% of cases [3]. Distinct subtypes of medulloblastoma have been identified. The large cell/anaplastic variety, in contrast to the classic and desmoplastic varieties, consists 10% of the cases and has particularly an aggressive clinical behavior [4].
Surgical resection of as much as possible of the intracranial tumor mass, followed by radiotherapy and chemotherapy, may cure up to 80% of patients, a rate that drops to 50% in patients with high-risk pathological features. Radiotherapy is directed to the cerebellum aiming to eliminate the primary tumor and to the craniospinal axis aiming to eradicate the micrometastatic disease. The recommended radiation dose to the cerebellum is 54–55.8 Gy and to the craniospinal axis 30–35 Gy, with a daily fractionation of 1.8 Gy [5]. Due to the high toxicity of craniospinal irradiation, lower doses of 23.4 Gy can be applied in combination with cisplatin-based systemic chemotherapy [5].
Due to the length of the irradiated body area, which extends from the top of the cranium to the coccyx, and the distinct volumes and depths of location of the brain and spinal cord, this inhomogeneous tissue target cannot be encompassed in a single radiotherapy field. 3D-planning for craniospinal irradiation comprises lateral fields to the cranium and one or two direct fields (according to the height of the patient) to the spine. As the maximum dimensions of a radiotherapy field of linear accelerators (LINACS) do not exceed 40 cm, inevitably, the direct beam to the cranium and spine is given with three adjacent fields in adults. Such three-area irradiation demands two gaps between the fields in order to avoid overlapping and over-dosage of the spinal cord to the points where two fields converge. Moving the gaps throughout the radiotherapy course is also essential to minimize the chances of spinal over or under-dosage within the gaps. Further, the direct fields deliver a higher dose to tissues overlying the spinal cord and a substantial dose to organs underlying the vertebra, like the esophagus.
In the current study we describe a radiotherapy technique using IGRT and VMAT that simplifies the cumbersome procedures for craniospinal irradiation of medulloblastoma patients.
Case Report
A 30-year-old woman diagnosed with anaplastic medulloblastoma, after local tumor subtotal surgical resection, was referred to our department for further treatment. Due to the high likelihood of recurrence, a combination of craniospinal radiotherapy with chemotherapy was offered to the patient. The radiotherapy schedule comprised whole brain and spinal axis irradiation with 17 fractions of 1.8 Gy (30.6 Gy total dose) followed by a booster dose of 12 fractions of 1.8 Gy (21.6 Gy) to increase the total dose to the cerebellum area to 50.4 Gy. Chemotherapy comprised a combination of cisplatin (50 mg/mL every two weeks) and temozolomide (70 mg/mL/daily, five days per week).
Radiotherapy was performed using the Infinity LINAC (Elekta, Stockholm, Sweden) with an Agility head (Elekta, Stockholm, Sweden) and multileaf collimator (MLC) featuring 0.5 cm leaves at the isocenter. A VMAT-technique was applied for craniospinal radiotherapy, while the booster dose to the occipital fossae was given with a 3D-conformal two-field technique. For computed tomography (CT) simulation, the patient was placed in a supine position with a thermoplastic mask immobilizing head and shoulders. Four triplets (one central and two laterals, each) of reference CT-markers were placed during the simulation, choosing areas with bony underlying structures, considered as less mobile, as follows; one on the thermoplastic mask at the level of the frontal head area, one at the level of the upper shoulder area, one at the level of the lowest thoracic area, and a fourth one at the level of the upper pelvis area. The head-marker was used for shifting the patient position to treatment isocenter, while the rest two triplets were used to adjust the position of the body on the LINAC table. The CT scan length was 100 cm and the slice thickness was 0.4 cm.
The CT set was transferred to Monaco Treatment Planning System (TPS) version 5.1 (Elekta CMS, Maryland Heights, MO, USA), to outline the volumes of interests. The structures outlined included the clinical target volume (CTV) and planning target volume (PTV) of the brain and spinal cord. Contouring of organs at risk (OAR: eyes, lenses of eyes, larynx, lungs, liver, kidneys, esophagus, and spleen) were performed. The CTV included the entire brain and spinal cord. The PTVBRAIN was created by adding a margin of 1 cm for brain CTV. For the PTVTHORACIC and PTVLUMBAR, lateral margins of 1.5 cm left and 1.5 cm right (to include spinal nerve roots) and margins of 1 cm anteriorly and 1 cm posteriorly were considered, followed by slice-by-slice corrections of the volume, where appropriate.
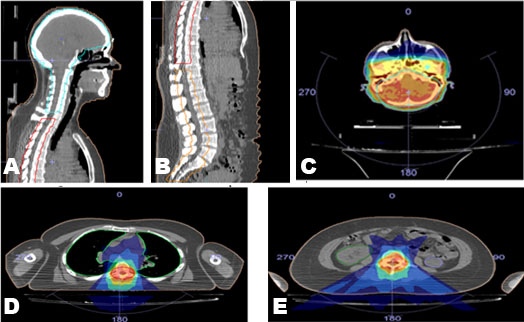
A 6 MV photon beam was used for the treatment planning in Monaco TPS version 5.1 (Elekta CMS, Maryland Heights, MO, USA). A VMAT technique and the Monte Carlo algorithm were used for isodose calculation. The prescription dose was 30.6 Gy in 17 fractions. The first challenge of this plan was to achieve the appropriate gap length using the VMAT technique. The main procedure to accomplish this aim was to separate the PTV into three PTVs; PTVBRAIN, PTVTHORACIC, and PTVLUMBAR. Among these structures, a gap of four slices (1.6 cm) was allowed, as shown in Figure 1A and Figure 1B. This decision was made after comparing the dose distribution within a gap of three, four, and five slices. The plan required three isocenters, and the isocentric shifts from reference CT markers were calculated from TPS. Each PTV had its own isocenter that was reached by moving manually the table from the head to the thoracic and then to the lumbar isocenter.
A second challenge was the week-by-week displacement of the gaps during the treatment, in order to displace the over- or underdosing occurring within the gap to more than one area of the spinal cord, so that the overall dose deviation in gaps was dissolved. For this reason, we produced three plans, one for each week, keeping the same isocenters and changing only the lengths of PTVs. The prescription of the plan of weeks 1 and 2 were 1.8 Gy × 6, and for week 3 was 1.8 Gy × 5 fractions. The shift of gaps between the fields was allowed only through the longitudinal dimension in order to avoid set up errors. Three cone-beam CTs were performed, for each one of the three PTVs, before each radiation treatment to check and adjust the position of the patient. Any adjustment was performed only at the anteroposterior and latero-lateral axis, while longitudinal corrections were not allowed.
During the first week, the PTVBRAIN involved the whole brain till the C6 vertebra, PTVTHORACIC involved T1 till the T11 vertebra, and PTVLUMBAR involved L1 vertebra till the coccyx. Every next week we increased the length of PTVTHORACIC by four slices, decreasing by four slices the rest of PTVs (brain and lumbar). For the irradiation of PTVBRAIN, we used two posterior partial arcs of 120° and for the other two PTVs two posterior partial arcs of 50°, as shown in Figure 1C, Figure 1D, Figure 1E.
The constraints used in the TPS are shown in Table 1. The statistical uncertainty of algorithm settings was set 1% per calculation. There was no need to apply any constraints for liver and spleen because the dose was very low at these critical organs. Dose volume histograms were used to evaluate the dose at OARs and PTVs. Figure 2A, Figure 2B, Figure 2C shows the total DVH of each plan.
The mean dose, max dose, and V20% (where Vx% = how much of organ volume receives 20% of total dose) of OARs are shown in Table 2. All results of Table 2 are within the tolerance limits reported by Normal Tissue Constraint Guidelines [6].
Discussion
As mentioned above, the most challenging part of this plan was to achieve an acceptable dose within the gaps among the PTVs. The minimum and maximum physical doses in the cold and hot spots per week, within the six gaps (three brain/thoracic and three thoracic/lumbar), were recorded, and the dose per fraction delivered to this point was calculated (Table 3). The biological equivalent dose to these points was, after that, calculated using the NTD formula, introduced by Macejewsky [7]. Normalized total dose formula is defined “as the total dose given in fractions of 2 Gy per day, which leads to the same biological effect as the actual treatment schedule for the endpoint under consideration.”
where D is the total dose of the altered fractionation scheme, d is the dose per fraction, and α/β is the ratio that provides the dose in Gy where cell killing from linear and quadratic components of the linear-quadratic equation is equal. For the spinal cord toxicity, an α/β-value of 2 Gy was considered.
As shown in Table 3, the spinal cord within the gaps receives a minimum and a maximum normalized total dose, 89% (26 Gy), and 120% (34.97 Gy) of the prescription, respectively. These doses are acceptable and safe for the patient. The minimum and a maximum NTD in the rest (outside the gaps) of spinal cord was calculated at 92.5% and 112.7% of the target dose, respectively.
Plan verification was made by Delta 4 (ScandiDos, Uppsala, Sweden). The Delta4 device is a cylindrical Poly Methyl MethAcrylate (PMMA) phantom that surrounds two crossing orthogonal planes with 1069 p-Si diodes. Figure 3 shows the gamma index of every plan, which agreed with the plan to within 3% of the isocentre dose and 0.3 cm.
The radiation treatment was accomplished without any delays. Chemotherapy was well tolerated with grade 1 nausea and development of grade 2 neutropenia that enforced a 1-week delay in the flow of chemotherapy. The patient has accomplished eight bi-weekly cycles of cisplatin followed by chemotherapy with temozolomide for six months. The patient is alive with no evidence of decease or radiotherapy toxicities fifteen months after the completion of radiotherapy [8].
Conclusion
Volumetric modulated arc therapy/image-guided radiation therapy for adults with medulloblastoma is feasible and provides high conformity of the distribution of radiation dose throughout the craniospinal axis, keeping the exposure of normal tissues to low levels. This technique can be applied even in adolescents and children, where the length of the fields is lower than the ones applied in adults, so that in younger ones two PTVs instead of three could suffice for the coverage of the target. As the length of the fields are shorter in children than in adults, the VMAT technique can be applied as reported in taller children or the technique can be further simplified in shorter ones where two PTVs could be applied instead of three. Non-toxic and oncologically acceptable dose fluctuations within the thoracic and lumbar gaps are obtained. Combination of the VMAT technique with cisplatin/temozolomide chemotherapy is well tolerated and is associated with mild neutropenia. Whether this radiotherapy combination with two approved drug combination is superior to the temzolomide alone or the triplet combination of older drugs (provarbazine, lomustine, and vincristine) deserves evaluation in clinical trials.
REFERENCES
1.
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64(2):83–103. [CrossRef]
[Pubmed]

2.
Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. Journal of Clinical Neuroscience 2012;19(11):1541–4. [CrossRef]
[Pubmed]

3.
Rochkind S, Blatt I, Sadeh M, Goldhammer Y. Extracranial metastases of medulloblastoma in adults: Literature review. J Neurol Neurosurg Psychiatry 1991;54(1):80–6. [CrossRef]
[Pubmed]

4.
Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 2016;131(6):803–20.
[Pubmed]

5.
National Comprehensive Cancer Network. [Available at: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf]

6.
Normal Tissue Constraint Guidelines. [Available at: http://www.remotecmd.com/uploads/3/1/0/6/31069079/normal_tissue_constraint.pdf]

7.
Maciejewski B, Taylor JM, Withers HR. Alpha/beta value and the importance of size of dose per fraction for late complications in the supraglottic larynx. Radiother Oncol 1986;7(4):323–6. [CrossRef]
[Pubmed]

8.
National Comprehensive Cancer Network. [Available at: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf]

SUPPORTING INFORMATION
Author Contributions
Christos A Nanos - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ioannis Abatzoglou - Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Michael I Koukourakis - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent to use anonymously clinical and laboratory data for research purposes was obtained before the onset of therapy.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Christos A Nanos et al.. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.