|
Case Report
Clinical and diagnostic imaging manifestations of Erdheim-Chester disease
1 Department of Internal Medicine, Kettering Health Network, Kettering, Ohio, USA
2 Department of Diagnostic Radiology, Kettering Health Network, Kettering, Ohio, USA
3 Kettering Cancer Center, Division of Medical Oncology and Hematology, Kettering, Ohio, USA
4 Wright State University Boonshoft School of Medicine, Dayton, Ohio, USA
5 Department of Ophthalmology, Kettering Health Network, Kettering, Ohio, USA
Address correspondence to:
Roland Gazaille
DO, Department of Diagnostic Radiology, Kettering Health Network, Kettering, Ohio,
USA
Message to Corresponding Author
Article ID: 100079Z10AT2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Thau A, Gazaille R, Calvo A, Borchers CE, Warwar R, Boyce W, Blake J. Clinical and diagnostic imaging manifestations of Erdheim-Chester disease. J Case Rep Images Oncology 2021;7:100079Z10AT2021.ABSTRACT
Introduction: Erdheim-Chester disease (ECD) is a rare form of non-Langerhans-cell histiocytosis which typically presents with bilateral masses due to retro-orbital deposition, xanthelasma of the eyelids, cardiopulmonary manifestations, along with sclerosis and cortical thickening of the long bones, particularly in the lower extremities.
Case Report: We present the case of a 58-year-old male who presented to a local emergency department with a one day history of severe dizziness, diaphoresis, dyspnea, and intermittent diplopia. Imaging demonstrated bilateral symmetric retrobulbar masses. Subsequent imaging as well as orbital and bone marrow biopsies lead to the diagnosis of ECD.
Conclusion: Erdheim-Chester disease is a rare form of non-Langerhans-cell histiocytosis. This case demonstrates classic clinical and imaging findings representative of ECD. Diagnostic imaging and pathologic findings play a vital role in the diagnosis and choice of therapy in patients with ECD. Although interferon is currently considered to be first-line therapy, targeted BRAF and MEK inhibitors hold promise for future direction.
Keywords: Erdheim-Chester disease, Histiocytosis, Proptosis, Retrobulbar masses
Introduction
Erdheim-Chester disease (ECD) is a rare form of non-Langerhans-cell histiocytosis [1]. Since its first description in 1930 by Jakob Erdheim and William Chester, approximately 1500 cases have been reported world-wide [2],[3]. Despite the majority of literature comprised by case reports, a predilection for males in their 5th to 7th decade of life with multisystem involvement that may include the central nervous system, skeletal, cardiovascular, renal, and dermatologic systems have been frequently reported [1],[4]. The presenting symptoms most commonly include diplopia, diabetes insipidus, dysarthria, headache, lower extremity bone pain, and imbalance, although many others have been described [1],[4].
Case Report
A 58-year-old male with a past medical history of obesity, hypertension, hyperlipidemia, diabetes mellitus type 2, hypertension, and asthma presented to the emergency department with severe dizziness, diaphoresis, shortness of breath, and intermittent diplopia. The patient stated that the dizziness had been worsening over the past several weeks and that the diplopia had started one day prior. The patient also admitted to experiencing headaches over the past year along with epigastric pain and cramping over the past day. The patient’s wife stated that she felt his eyes had been “bulging” over the past six months. The patient’s review of systems was additionally significant for right knee and leg pain, which had been ongoing for five years.
On ophthalmic examination, the patient’s visual acuity was 20/30 OU. The patient exhibited a mild deficit in abduction bilaterally. Funduscopic examination was normal. The patient exhibited bilateral symmetric proptosis (Hertel exopthalmometry 26 mm OU). Bilateral xanthelasma was noted over the medial upper eyelids (Figure 1 and Figure 2), which developed over the prior year. The patient underwent a right retrobulbar 20 gauge core needle biopsy approximately two weeks later.
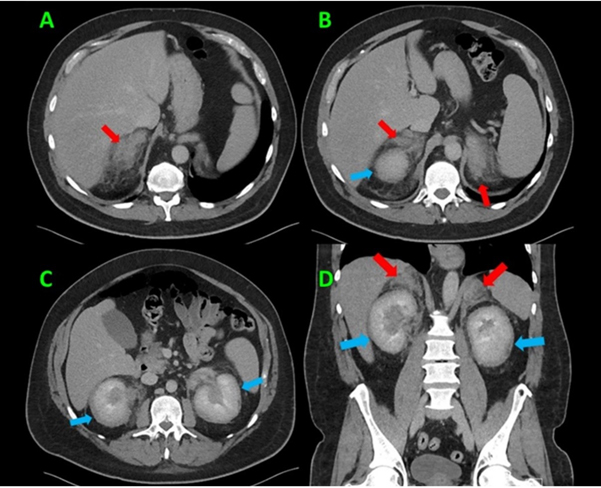
A few days following the biopsy, the patient returned to the emergency department with new onset fever and chills along with continued vision changes, headaches, and abdominal pain. The results from the biopsy were still pending. The patient was found to have a temperature of 102.9°F, tachycardia with heart rate 131 bpm, and tachypnea with a respiratory rate of 25 bpm. Aside from bilateral proptosis the remainder of the patient’s physical examination was unremarkable. Laboratory work-up showed leukocytosis of 19,000/mm3 and a procalcitonin level of 7.61 μg/L. Initial imaging studies included a computed tomography (CT) of the head and orbits with contrast (Figure 3) which revealed bilateral exophthalmos due to symmetric bilateral retrobulbar masses encasing the bilateral optic nerves. The working differential diagnosis was lymphoma, leukemia, metastatic disease, or some other type of infiltrative neoplastic or inflammatory process. Given the patient’s complaint of abdominal pain, a CT of the abdomen and pelvis with contrast (Figure 4) was obtained and demonstrated infiltrative changes and enlargement of the bilateral adrenal glands and kidneys. The patient’s chest radiographs were unremarkable.
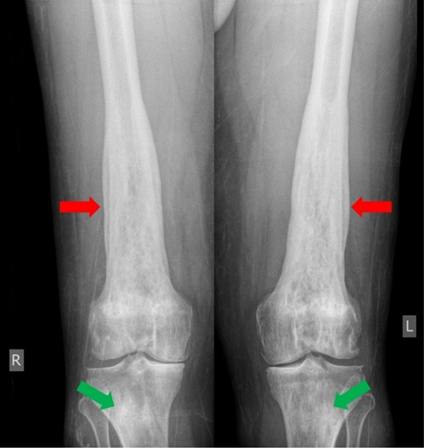
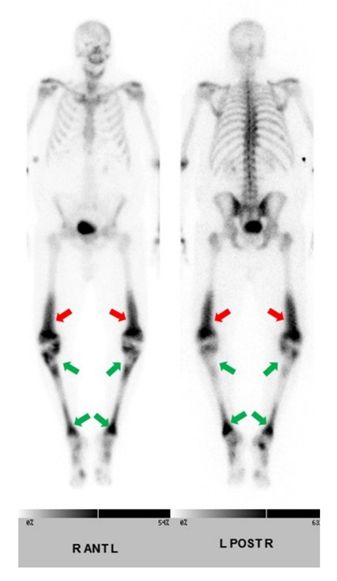
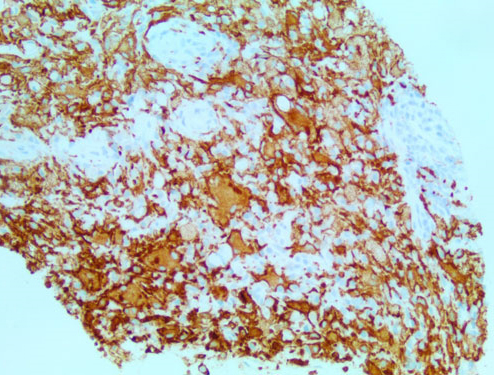
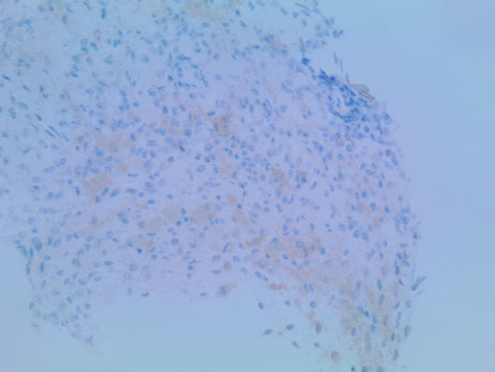
The patient’s presentation of fever, tachycardia, tachypnea, and leukocytosis was concerning for sepsis, however no source of infection was identified on the initial work-up. His lactic acid level was 1.9 mmol/L. He was started on empiric antibiotic therapy with Vancomycin [Vancocin] and Piperacillin-Tazobactam [Zosyn]. Blood and urine cultures were ordered. Although the CT scan did raise some initial concerns for acute pyelonephritis in the setting of a clinical suspicion of sepsis, the patient was asymptomatic without any dysuria, suprapubic tenderness, or changes in frequency of urination. His urinalysis was unremarkable at the time of admission. The patient did not respond to antibiotics which were eventually discontinued. Due to concerns that this could be some type of neoplastic process, a skeletal survey was ordered which revealed a symmetric pattern of cortical thickening and sclerosis involving the bilateral distal femoral metadiaphyses (Figure 5). The bilateral proximal tibial metaphyses showed similar but less pronounced changes. A bone scan revealed a similar pattern of bilateral and symmetric abnormal uptake of radiotracer in the bilateral distal femoral and proximal tibial metadiaphyses (Figure 6). A CT-guided biopsy of the distal right femoral metadiaphysis (not shown) was performed and demonstrated sclerotic bone with histiocyte infiltration, consistent with ECD. The core biopsy of the right retro-orbital mass demonstrated foamy histiocytes and macrophages (Figure 7). The histiocytes were positive for CD163 (Figure 8), CD68, and factor XIII. Cyclin D1 was strongly positive in a subset of histiocytes and endothelial cells. Multinuclear giant cells (Touton cells) were not reported. The histiocytes were weakly positive for BRAF (Figure 9). The patient was started on Dexamethasone [Decadron] 4 mg intravenous (IV) every 6 hours with the hope of reducing any swelling or inflammation involving the optic nerves. The patient was started on pegylated interferon. After four infusions, the patient continued to experience difficulties with his vision. At that time, it was reported that there was an insufficient amount of material from the bone marrow biopsy to perform BRAF testing. The patient was referred to a University-based ECD clinic where he was started on cobimetinib after having undergone two months of pegylated interferon infusions. He did not tolerate the cobimetinib well and it was discontinued after one month. The patient was found to have progression of his disease on a follow-up magnetic resonance imaging (MRI) of the orbits at which time pegylated interferon was reinitiated. The toxicity that the patient has experienced from the pegylated interferon has been manageable and has included arthralgias, edema, and a headache. He continues to follow-up with the University-based EDC clinic.
Discussion
Due to its infrequent presentation and non-specific symptoms, ECD presents a diagnostic challenge for the clinician. In 2013, Haroche et al. suggested diagnostic criteria which include both histologic and radiologic components [5]. Pathologic finding characteristics of ECD include infiltration of nearly any tissue with foamy histiocytes and Touton giant cells with xanthomatous features [1],[4],[5]. Immunochemistry findings include CD68, CD163, and factor XIIIa positivity as well as CD1a and CD207 negativity [1],[3],[4],[5]. Variability in S100 exists, with approximately 20% positivity [1],[3],[4],[5].
Radiologic findings have an important role in the diagnosis of ECD. Nearly 95% of patients have been reported with bony involvement, noted by a pathognomonic radiologic finding of osteosclerosis involving the metadiaphyseal regions of the bones around the knees [6],[7]. Additionally, nearly 50% of patients describe lower extremity bone pain, which may prompt imaging studies to be performed [6],[7]. Screening for suspected ECD has been suggested with whole-body bone scintigraphy or 18F-fluorodeoxyglucose positron emission tomography-computed tomography, the latter of which may be beneficial for guiding tissue biopsy.
The understanding of the biomolecular pathogenesis of ECD has been rapidly progressing in recent years. These advancements provide opportunity for targeted therapies. Notably in 2012, oncogenic BRAF V600E mutations were recognized in ECD histiocytes [8]. With over 60% of cases positive for this mutation, the use of BRAF targeted therapy has emerged [3]. Vemurafenib [Zelboraf], an inhibitor specific to BRAF V600E, has demonstrated an impressive rate of remission from 43% to 100% [3]. As not all cases of ECD have demonstrated a BRAF mutation, inhibition of other signaling pathways have been attempted. Cobimetinib [Cotellic], a MEK inhibitor, has also been attempted with similar results [3]. Combination therapy of Vemurafenib [Zelboraf] and Cobimetinib [Cotellic] has been attempted with disease regression noted in all 9 cases identified [9]. Unfortunately, these therapies are not without side effects and interruption of therapy resulted in disease relapse in 75% of cases at six months [9]. Although these cases demonstrate promise for the future of targeted therapies in ECD, at this time there have been too few cases to draw meaningful results. First-line therapy remains interferon-alpha therapy, as the largest retrospective cohort-study identified it as an independent predictor of survival [10]. This study demonstrated a five-year survival rate on interferon-alpha therapy at 70%. At this time, there is little data to demonstrate the survival-benefit of BRAF or MEK targeted therapies.
Conclusion
Erdheim-Chester disease is a rare form of non-Langerhans-cell histiocytosis. This case demonstrates classic clinical and imaging findings representative of ECD. Diagnostic imaging and pathologic findings play a vital role in the diagnosis and choice of therapy in patients with ECD. Although interferon therapy is currently first-line, targeted BRAF and MEK inhibitors hold promise for future direction.
REFERENCES
1.
Cives M, Simone V, Rizzo FM, et al. Erdheim-Chester disease: A systematic review. Crit Rev Oncol Hematol 2015;95(1):1–11. [CrossRef]
[Pubmed]

2.
3.
Papo M, Emile JF, Maciel TT, et al. Erdheim-Chester disease: A concise review. Curr Rheumatol Rep 2019;21(12):66. [CrossRef]
[Pubmed]

4.
Marinelli JP, Peters PA, Vaglio A, Van Gompel JJ, Lane JI, Carlson ML. Skull base manifestations of Erdheim-Chester disease: A case series and systematic review. Neurosurgery 2019;85(4):E693–701. [CrossRef]
[Pubmed]

5.
Haroche J, Arnaud L, Cohen-Aubart F, et al. Erdheim-Chester disease. Rheum Dis Clin North Am 2013;39(2):299–311. [CrossRef]
[Pubmed]

6.
Goyal G, Young JR, Koster MJ, et al. The Mayo Clinic Histiocytosis Working Group consensus statement for the diagnosis and evaluation of adult patients with histiocytic neoplasms Erdheim-Chester disease, Langerhans cell histiocytosis, and Rosai-Dorfman disease. Mayo Clin Proc 2019;94(10):2054–71. [CrossRef]
[Pubmed]

7.
Estrada-Veras JI, O’Brien KJ, Boyd LC, et al. The clinical spectrum of Erdheim-Chester disease: An observational cohort study. Blood Adv 2017;1(6):357–66. [CrossRef]
[Pubmed]

8.
Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood 2012;120(13):2700–3. [CrossRef]
[Pubmed]

9.
Cohen Aubart F, Emile JF, Carrat F, et al. Targeted therapies in 54 patients with Erdheim-Chester disease, including follow-up after interruption (the LOVE study). Blood 2017;130(11):1377–80. [CrossRef]
[Pubmed]

10.
Cohen-Aubart F, Emile JF, Carrat F, et al. Phenotypes and survival in Erdheim-Chester disease: Results from a 165-patient cohort. Am J Hematol 2018;93(5):E114–7. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Avrey Thau - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Roland Gazaille - Conception of the work, Design of the work, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Alejandro Calvo - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Christina E Borchers - Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ronald Warwar - Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
William Boyce - Conception of the work, Design of the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Joseph Blake - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Avrey Thau et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.