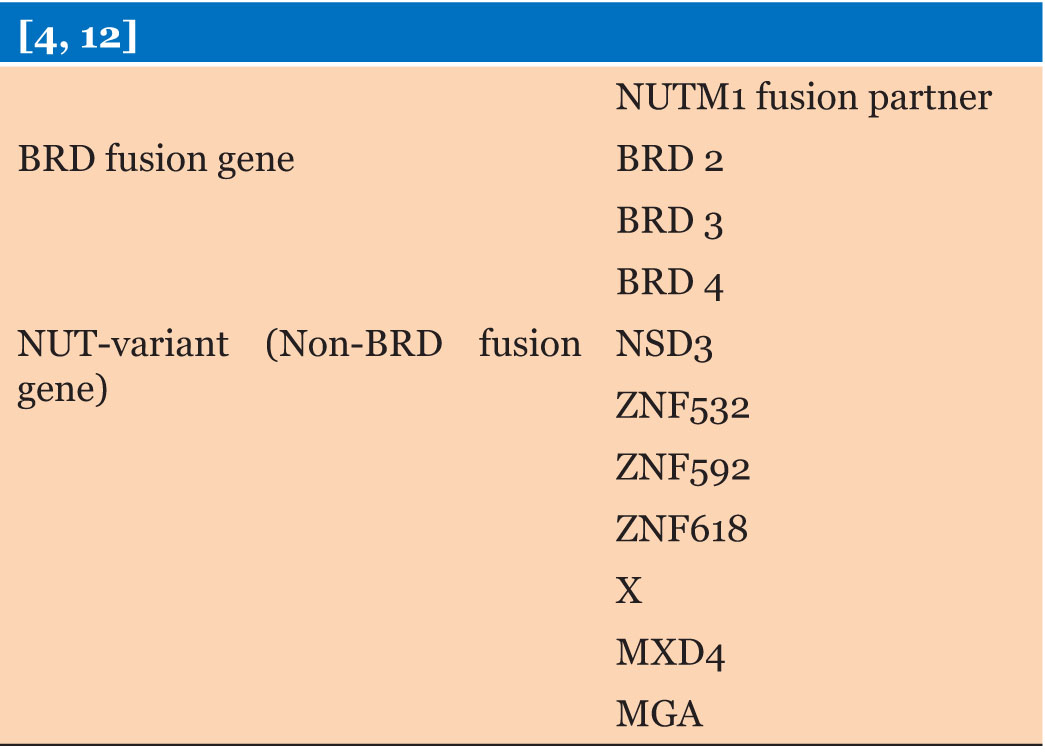
|
Case Report
Rapid-onset and fatal pneumonitis from trametinib treatment of non-small cell lung cancer: A case report
1 James P Wilmot Cancer Institute, Division of Hematology/Oncology, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA
2 James P Wilmot Cancer Institute, Division of Radiation Oncology, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA
Address correspondence to:
Seyed Mohammad Abedi
James P Wilmot Cancer Institute, Division of Hematology/Oncology, University of Rochester School of Medicine and Dentistry, Rochester, New York,
USA
Message to Corresponding Author
Article ID: 100077Z10SA2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Abedi SM, Lekkala M, Chen Y, Baumgart M, Patel A. Rapid-onset and fatal pneumonitis from trametinib treatment of non-small cell lung cancer: A case report. J Case Rep Images Oncology 2021;7:100077Z10SA2021.ABSTRACT
Introduction: Lung cancer remains the leading cause of death in both men and women worldwide. Oral targeted therapy remains the recommended first-line approach for those with actionable mutations. The combination of trametinib and dabrafenib has shown durable responses as both a first line and second line treatment in patients with non-small cell lung cancer (NSCLC) with a BRAFV600E mutation. Respiratory complications with trametinib have rarely been documented, with an incidence of less than 2%.
Case Report: A 58-year-old former female smoker who presented with dyspnea on exertion and was found to have a right hilar mass. The mass was biopsied and found to be a poorly differentiated carcinoma consistent with NSCLC. Tumor proportion score (TPS) was 100% for programmed death-ligand 1 (PD-L1) expression, and molecular analysis confirmed a BRAFV600E mutation. She was started on treatment with dabrafenib 150 mg twice daily with trametinib 2 mg once daily. After ten days, she developed fever followed by leukocytosis and hypoxia. Chest imaging was suggestive of pneumonitis, and she was initiated on high-dose steroids and antibiotics. Her cultures remained negative, though she was unable to be weaned from high-flow oxygen. She transitioned to hospice care several days later and subsequently passed in another 12 days.
Conclusion: Trametinib-induced interstitial pneumonitis, while a relatively rare occurrence, can become rapidly life-threatening and should prompt immediate cessation of the medication followed by urgent supportive care measures.
Keywords: Drug hypersensitivity, Lung neoplasms, NSCLC, Pneumonitis, Trametinib
Introduction
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer-related deaths worldwide. Significant progress has been made in understanding the molecular pathways that drive malignancy in NSCLC. BRAF mutations have been observed in 1–3% of NSCLC and are usually seen in patients with a smoking history [1],[2]. Dabrafenib and trametinib as a combination has the potential to elicit durable responses in both the first line and second line studies in NSCLC with BRAFV600E (Val600Glu) [3]. Respiratory complications are extremely rare in patients treated with combination dabrafenib and trametinib, and more often thought to be due to trametinib. In clinical trials for the treatment of metastatic melanoma that led to the approval of the mitogen-activated protein kinase (MEK) inhibitor, the incidence was <2%, with median time to presentation of about 160 days (60–172 days). Even the 5-year outcome studies in melanoma did not show an increased incidence of pneumonitis [4]. Here, we report a case where a patient developed fatal interstitial pneumonitis within 10 days of starting treatment with trametinib.
Case Report
Our patient was a 58-year-old female with a past medical history of seizure disorder controlled on phenobarbital, hypothyroidism controlled on levothyroxine, and tobacco abuse with a 60 pack year history who quit 7 years prior to presentation, initially presented to the emergency department with dyspnea on exertion associated with chest pain radiating to her back. A computed tomography (CT) scan with angiography of the chest was performed and showed a right hilar mass that measured 7 cm by 4 cm. A positron emission test (PET) scan with CT showed 18F-fluorodeoxyglucose (FDG) avidity in this lesion with a standardized uptake value of 18, hypermetabolic mediastinal lymphadenopathy, a right lower lobe pulmonary lesion, and abdominal lymphadenopathy suggestive of metastatic disease. Histologic workup showed a poorly differentiated carcinoma with PD-L1 tumor proportion score of 100%. Molecular analysis of the tumor confirmed BRAFV600E mutation. Subsequently she was started on dabrafenib 150 mg twice daily with trametinib 2 mg once daily. Eight days later, she developed a fever with no clinical nidus for infection. She was treated with nonsteroidal anti-inflammatory drugs and acetaminophen for treatment of therapy-related pyrexia. Ten days later she was admitted with fever, leukocytosis, and hypoxia. Chest X-ray on admission showed right upper lung opacities concerning for pneumonia and she was treated with antibiotics, however, no organisms were identified from blood cultures, and she was discharged with oxygen support with two liters nasal cannula. After hospital discharge, dabrafenib was restarted at 50 mg twice daily with a plan to restart trametinib at a dose of 1 mg daily in seven days time. Unfortunately, trametinib was never restarted as the patient was re-admitted with worsening hypoxia, and CT scan of the chest was suggestive of pneumonitis (Figure 1). There was also evidence of new bilateral upper lobe airspace opacities concerning for focal infection. Interval decrease of mediastinal or hilar lymphadenopathy was also seen, likely due to treatment response.
She was initiated on antibiotics and steroids. Antibiotics were subsequently stopped when cultures were negative and imaging was representative of pneumonitis. High dose steroids were continued with Solu-medrol 125 mg every 8 hours. She had persistent, severe hypoxia requiring high flow oxygen of 50 liters, which was unable to be weaned over the course of her hospital stay. After 10 days, she enrolled in hospice care and passed peacefully 12 days after.
Discussion
Trametinib is an oral, reversible, and highly selective allosteric inhibitor of mitogen-activated protein kinase (MEK) 1/2 that effectively suppresses extracellular signal-regulated kinases (ERK), the activation of which can lead to degradation of pro-apoptotic proteins [5]. Combination therapy with the BRAF inhibitor dabrafenib and trametinib has elicited durable responses in both the first line and second line studies in patients with NSCLC with BRAFV600E [3].
Pneumonitis has been rarely reported with use of trametinib, though when it has occurred, all patients required hospitalization. In a phase 1b study of trametinib with gemcitabine in advanced solid tumors, it was shown that at a dose of 2 mg, there was one case of grade 1/2 and one case of grade 3 pneumonitis seen among the 21 patients in the study [6]. In the PACMEL study, trametinib was used in combination with paclitaxel for treatment of melanoma, and grade 1/2 pneumonitis was reported in 1 of 15 patients [5]. In a study evaluating trametinib plus docetaxel or pemetrexed in patients with advanced NSCLC no significant pneumonitis was noted [7]. In another study, trametinib plus dabrafenib was used in treatment-naïve patients with BRAFV600E-mutant metastatic NSCLC [3]. Of the 36 enrolled patients, there are no reported cases of pneumonitis; however, there were 8 (22%) patients with cough and 4 (11%) with dyspnea.
The possibility of dabrafenib-induced pneumonitis was investigated. It appears that respiratory complications are extremely rate with BRAF inhibitors such as dabrafenib or vemurafenib [8]. A study of trametinib plus dabrafenib in melanoma patients showed a 2.4% risk of interstitial lung disease (ILD) or pneumonitis associated with trametinib while no cases were reported in the dabrafenib arm [9],[10]. These data also reinforce trametinib as the likely cause of pneumonitis in our patient.
While rare, median time to onset of reported cases of pneumonitis was 160 days; however, now we have evidence that rapid onset of severe interstitial pneumonitis within 30 days can occur in patients on trametinib. For this reason, it is imperative to keep a high index of suspicion for patients on this agent presenting with signs and symptoms suggestive of pneumonitis. Reducing the dose of trametinib for grade 1 pneumonitis or temporarily holding trametinib for grade 2 pneumonitis and resuming at a reduced dose when symptoms improve to grade 1 is reasonable. For cases of grades 3 and 4 For cases of grade 3 and 4 toxicity, trametinib should be permanently discontinued. Cases of grade 2 or higher pneumonitis will also require high-dose corticosteroids, chest CT scans, and possibly hospitalization for respiratory care. For patients being treated with trametinib in combination with dabrafenib, dabrafenib can be resumed [8].
Conclusion
The possibility of pneumonitis must be considered for patients on dabrafenib and trametinib who develop a new or worsening cough or dyspnea. This should be investigated with plain chest X-ray or chest CT scan, and treatment should be halted at least temporarily if grade 2 through grade 4 pneumonitis is suspected or identified. Patients often require hospitalization and aggressive supportive measures, as toxicity can be fatal.
REFERENCES
1.
Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol 2014;25(1):138–42. [CrossRef]
[Pubmed]

2.
Sequist LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol 2011;22(12):2616–24. [CrossRef]
[Pubmed]

3.
Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol 2017;18(10):1307–16. [CrossRef]
[Pubmed]

4.
Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019;381(7):626–36. [CrossRef]
[Pubmed]

5.
Coupe N, Corrie P, Hategan M, et al. PACMEL: A phase 1 dose escalation trial of trametinib (GSK1120212) in combination with paclitaxel. Eur J Cancer 2015;51(3):359–66. [CrossRef]
[Pubmed]

6.
Infante JR, Papadopoulos KP, Bendell JC, et al. A phase 1b study of trametinib, an oral Mitogen-activated protein kinase kinase (MEK) inhibitor, in combination with gemcitabine in advanced solid tumours. Eur J Cancer 2013;49(9):2077–85. [CrossRef]
[Pubmed]

7.
Gandara DR, Leighl N, Delord JP, et al. A phase 1/1b study evaluating trametinib plus docetaxel or pemetrexed in patients with advanced non-small cell lung cancer. J Thorac Oncol 2017;12(3):556–66. [CrossRef]
[Pubmed]

8.
Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol 2015;7(2):122–36. [CrossRef]
[Pubmed]

9.
Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367(18):1694–703. [CrossRef]
[Pubmed]

10.
Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367(2):107–14. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Seyed Mohammad Abedi - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Manidhar Lekkala - Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yuhchyau Chen - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Megan Baumgart - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Arpan Patel - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Seyed Mohammad Abedi et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.