| Table of Contents |  |
|
Case Report
| ||||||
| Acute pancreatitis induced by cisplatin-etoposide regimen | ||||||
| Abdulqadir J. Nashwan1, Hind H. Elmalik2, Sindhumole L. K. Nair1, Mohamed A. Yassin3 | ||||||
|
1Nurse Educator, Hamad Medical Corporation – Nurse Educator, Department of Nursing Education & Research, National Center for Cancer Care and Research (NCCCR), Doha, Qatar.
2Oncology Consultant, Hamad Medical Corporation – Oncology Consultant, Department of Medical Oncology, National Center for Cancer Care and Research (NCCCR), Doha, Qatar. 3Hematology Consultant, Hamad Medical Corporation – Hematology Consultant, Department of Hematology and BMT, National Center for Cancer Care and Research (NCCCR), Doha, Qatar. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article: |
| Nashwan AJ, Elmalik HH, Nair SLK, Yassin MA. Acute pancreatitis induced by cisplatin-etoposide regimen. J Case Rep Images Oncology 2016;2:61–65. |
|
Abstract
|
|
Introduction:
Drug-induced pancreatitis is less prevalent compared to other causes of acute pancreatitis, the incidence ranges between 0.1–2% of acute pancreatitis. Several reports are suggesting the association of pancreatitis with cisplatin but etoposide has not been reported to associate with pancreatitis.
Case Report: A 25-year-old, Filipino lady presented with Stage IV cervical cancer with neuroendocrine features. She was started on chemotherapy regimen of cisplatin and etoposide. She tolerated the six cycles of chemotherapy, and she was planned to start concurrent chemoradiation. However, due to persistently elevated levels of serum creatinine, she could not receive cisplatin. During radiotherapy, she has developed acute pancreatitis. The common causes of acute pancreatitis were ruled out; given the time course, it was assumed that the chemotherapeutic agents; cisplatin and etoposide might be suggested risk factors for the development of pancreatitis in our patient. Conclusion: Early identification of acute pancreatitis helps in instituting effective treatment. Therefore, knowledge regarding acute pancreatitis related to cisplatin has utmost significance. | |
|
Keywords:
Acute Pancreatitis, Cisplatin, Etoposide, Neuroendocrine cervical cancer
| |
|
Introduction
| ||||||
|
Small cell neuroendocrine carcinoma of cervix (SCNEC) is an uncommon tumor, accounting less than 5% of all cervical cancer cases. It is characterized by high metastatic rates, resulting in a poor prognosis [1]. It is highly aggressive and managed with a multimodality approach. Chemotherapy and radiotherapy are used for advanced cases. Drug-induced pancreatitis is not very common, and overall incidence ranges from 0.1–2% of all acute pancreatitis cases [2]. Most of these cases are mild and self-limiting. For many drugs, the pathogenesis is not completely understood [2]. However, drug-induced pancreatitis is usually a diagnosis of exclusion. Chemotherapeutics have also been known to be associated with acute pancreatitis. Acute pancreatitis induced by both cisplatin and etoposide regimens have never been reported. However, there are few studies published on cisplatin-induced acute pancreatitis [3]. We report a case of acute pancreatitis might be related to chemotherapy of cisplatin and etoposide. | ||||||
|
Case Report
| ||||||
|
A 25-year-old, single, sexually active, Filipino female presented with irregular vaginal bleeding for six months and fishy vaginal discharge. Cervical biopsy consistent with poorly differentiated carcinoma with neuroendocrine features. Magnetic resonance imaging (MRI) pelvis revealed a large exophytic polypoid tumor measuring 8x8x7.2 cm arising from the exocervix distending the vaginal fornix and protruding up to lower third of vagina with no definite vaginal wall invasion or parametrial involvement. No pelvic lymphadenopathy. Overall FIGO (International Federation of Gynecology and Obstetrics) staging corresponds to stage IB2. Positron emission tomography (PET) scan showed intense hypermetabolism in the known cervical cancer. FDG (Fluorodeoxyglucose)-avid right lung nodule suspicious for metastasis. The patient had undergone six cycles of chemotherapy (cisplatin 25 mg/m2 and etoposide 100 mg/m2), and she had tolerated well during the course. After the chemotherapy, concurrent chemoradiation (cisplatin 25 mg/m2 once in a week) was planned for her. But radiotherapy alone was started as patient's creatinine level rose to 138 (53–97 µmol/L) and persisted as high during radiotherapy. During her 1st radiotherapy visit, she complained of nausea and vomiting in the early morning and also complained of upper abdominal pain. Patient advised following-up with ultrasonography (USG) report. After one week, she again presented with severe increasing epigastric pain radiated to the back with right hypochondriac pain associated with vomiting related to eating heavy meals. Tenderness was noticed in the right upper and central abdomen, ultrasonography was not done. Patient has no history of cough, fever or shortness of breath or skin rashes, no bowel motion disturbances. Patient's labs showed elevated serum lipase 163 (13–60 U/L), with mild elevation in the liver enzymes ALT 48 (0–30 U/L); AST 78 (0–31 U/L)) and high LDH level 2185 (135–214 U/L), serum creatinine level 122 (53–97 µmol/L), serum calcium 2.27 (2.05–2.55 mmol/L), C-reactive protein (CRP) 58 (0–5 mg/L), cisplatin serum level was not done. Abdominal radiography (abdominal X-ray) revealed no significant bowel dilatation or air-fluid level. Ultrasonography (USG) showed cirrhotic changes in the liver with metastatic deposits; no intrahepatic biliary radical dilatation; Gallbladder contracted, however, multiple stones, largest one measuring 9 mm in size noted; Common bile duct was not dilated. A computed Tomography (CT) scan of the abdomen was not done as the patient had renal impairment. The patient was placed on nil per oral status (NPO) and treated with intravenous ?uids and analgesics. With supportive therapy, her pain subsided and her lipase normalized within four days and the patient was started an oral diet. The patient was diagnosed to have an acute pancreatitis with clinical signs and symptoms and laboratory values. Her lipase level was 163 U/L. Elevated lipase levels are more specific to the pancreas than high amylase levels. High CRP value (58 mg/L) indicated for severe acute pancreatitis. Abdominal radiography excluded the cause of acute pancreatitis from a perforated duodenal ulcer. Liver associated enzyme levels were tested to look for gallstone-associated pancreatitis. An ALT level higher than 150 U/L suggests gallstone pancreatitis, but there was an only mild elevation in liver enzymes. Her ultrasonography scan showed evidence of gallstones, but common bile duct was not dilated. Hence, gallstone was excluded from the expected causes. Other common causes of pancreatitis were also excluded. She was abstinent of alcohol, had normal serum calcium, had no family history of pancreatitis or hyperlipidemia, and had no history of trauma. She was not on any concomitant medications for a long period too. By exclusion, the diagnosis of drug-induced pancreatitis secondary to chemotherapy was made. | ||||||
|
Discussion
| ||||||
|
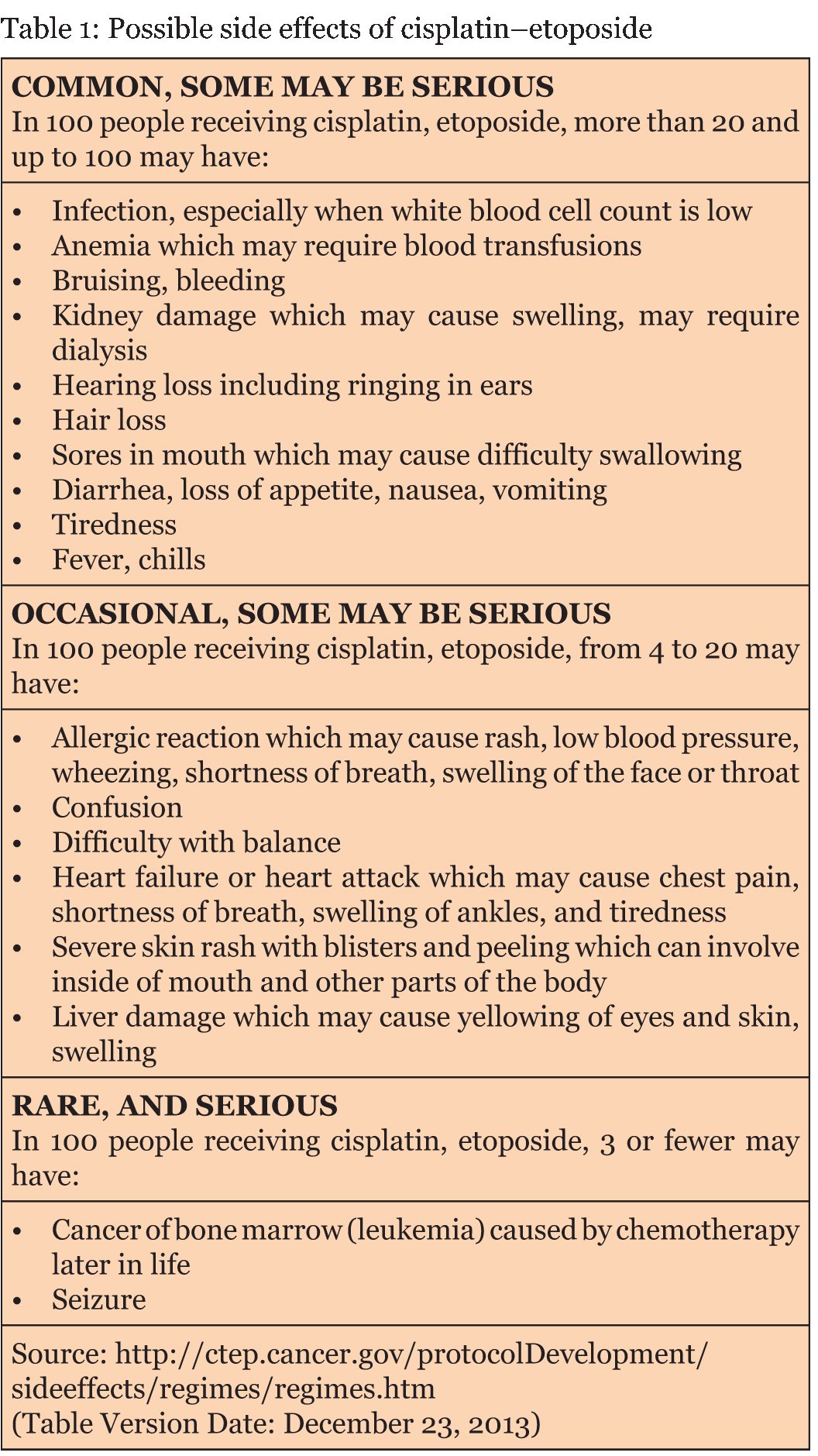
Cervical cancer is one of the most common cancers affecting women worldwide. Neuroendocrine tumors account for only 2% of all cervical cancers [4]. These highly aggressive tumors have a prognosis much worse than that for stage comparable with poorly differentiated squamous cell carcinoma of the cervix [4]. Our patient was started on chemotherapy regimen consisted of cisplatin and etoposide. This particular regimen was based on the Society of Gynecologic Oncology (2011) [5] and treatment guidelines of high-grade lung and extrapulmonary NEC provided by the North American Neuroendocrine Tumor Society (NANETS) [6]. Acute pancreatitis is a surgical emergency characterized by upper abdominal pain, nausea, and vomiting, with elevated serum amylase and or lipase. The pathogenesis is suspected to involve enzymatic autodigestion of the pancreas, gallstones and alcohol account for over 80% of cases [2] [3] [4] [5] [6] [7]. Hypertriglyceridemia, hypercalcemia, drugs, and infection are rarer causes, drugs are implicated in only up to 2% of cases [2]. The identification of an acute pancreatitis is known to be fatal, with a mortality rate of almost 10% if severe disease is not properly diagnosed, and if effective treatment modalities are not initiated immediately [8]. Even in cases in which diagnosis and treatment are rapid, acute pancreatitis can prove life-threatening. According to the authors, clinical observations; many drugs or chemotherapy-induced acute pancreatitis are under-reported or misdiagnosed with other related conditions. The identification of the cause of acute pancreatitis is very crucial to initiate best possible care to ease early recovery and to avoid further occurrence. Our patient did not have a history of alcohol intake within the few months before hospitalization. Also, she had no history of any medical conditions that could lead to acute pancreatitis. Abdominal ultrasound revealed gallstones. However, there is no biliary ductal dilatation involving the common bile duct. Medications are also known to cause pancreatitis; with a frequency of 1.4–2.0% [7]. The most common toxicities associated with cisplatin and etoposide (CE) regimen are nausea and vomiting, ototoxicity, nephrotoxicity, myelosuppression, and electrolyte disturbances. Side effects of cisplatin-etoposide regime are summarized in Table 1 [9]. Acute pancreatitis associated with cisplatin-etoposide regimen has never been previously reported. Based on the outcome of rechallenge/dechallenge test, exclusion of other possible causes of pancreatitis, cisplatin was classified under the category of definitive [10] [11] cause of drugs for developing pancreatitis [11][12]. In this case, based on Naranjo score [13], Cisplatin could be a possible cause for the development of acute pancreatitis and the relation between etoposide and acute pancreatitis is doubtful only [10] [11]. Etoposide is a semisynthetic derivative of podophyllotoxin that binds to and inhibits topoisomerase II, and DNA leads to cell death. Cisplatin has been used to treat a range of tumors including head and neck, lung, breast, ovarian and cervical cancers [14]. Cisplatin is damaging DNA in tumor cells through the creation of covalent bonds with purine bases [15]. According to Jamieson and Lippard, cisplatin is classified as Class II drugs in terms of association with pancreatitis (>ten but <20 reported cases of acute pancreatitis with or without positive re-challenge) [16]. While another review had classified cisplatin as a Class IV (based on single reported cases) [17]. Total of five cases were reported the possible association between cisplatin and development of acute pancreatitis [18] [19][20]. Cisplatin, etoposide regimen is most frequently used to treat small cell carcinoma of uterine cervix [21] [22] [23] . Over the period of 100 days, the patient has received a total dose of 730 mg of cisplatin and 2900 mg of etoposide. The cumulative effect of cisplatin and etoposide could be the cause of acute pancreatitis in this patient. The authors recommend to stop or change the chemotherapy if the patients develop acute pancreatitis with either cisplatin or etoposide or combination of both and to initiate effective treatment as quickly as possible. | ||||||
| ||||||
|
| ||||||
|
Conclusion
| ||||||
|
A young lady with small cell neuroendocrine cervical cancer who received treatment with cisplatin and etoposide regimen for six cycles presented with acute pancreatitis. There are many case reports and studies suggesting that cisplatin, which is widely used and promoted for the treatment of lung, breast, and cervical cancers, could develop acute pancreatitis. However, to date, there has been no report of etoposide-induced acute pancreatitis. This study is the first one highlighting the fact that the patient undergoing treatment with cisplatin and etoposide need to be warned about the potential development of pancreatitis. | ||||||
|
Acknowledgements
| ||||||
|
We acknowledge the patient who inspired us to disseminate this article. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Abdulqadir J. Nashwan – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Hind H. Elmalik – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Sindhumole L. K. Nair – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Mohamed A. Yassin – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2016 Abdulqadir J. Nashwan et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|
|
About The Authors
| |||
| |||
| |||
| |||
| |||