Case Report
A case of primary squamous cell carcinoma of the parotid gland and review of the literature
1 School of Stomatology, Shanxi Medical University, Taiyuan, Shanxi, China
1 Shanxi Province Key Laboratory of Oral Diseases Prevention and New Materials, Taiyuan, Shanxi, China
2 School of Stomatology, Shanxi Medical University, Taiyuan, Shanxi, China
2 First Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
3 School of Stomatology, Shanxi Medical University, Taiyuan, Shanxi, China
4 School of Stomatology, Shanxi Medical University, Taiyuan, Shanxi, China
4 Shanxi Province Key Laboratory of Oral Diseases Prevention and New Materials, Taiyuan, Shanxi, China
5 School of Stomatology, Shanxi Medical University, Taiyuan, Shanxi, China
Address correspondence to:
Xinrong Nan
First Hospital of Shanxi Medical University, Taiyuan, Shanxi,
China
Access full text article on other devices

Access PDF of article on other devices

Article ID: 100128Z10JZ2024
doi: 10.5348/100128Z10JZ2024CR
How to cite this article
Zhao J, Nan X, Zhou C, Jiang N, Tian L. A case of primary squamous cell carcinoma of the parotid gland and review of the literature. J Case Rep Images Oncology 2024;10(1):7–12.ABSTRACT
Introduction: Primary parotid squamous cell carcinoma (PPSCC) is a rare malignant epithelial tumor originating in the parotid gland with complex diagnostic procedures and a poor prognosis. The etiology, tissue origin, and biological behavior of the disease are currently unknown due to its underreporting. It is crucial to differentiate this type of squamous cell carcinoma (SCC) of the parotid gland from other salivary gland tumors because of its widespread invasiveness, which leads to high mortality.
Case Report: This article reports a case of a 62-year-old male patient in whom histopathological examination confirmed that the tumor was squamous cell carcinoma. Since the patient had no other main source, the ultimate diagnosis was the primary SCC of the parotid gland. The patient is currently receiving routine follow-up care and has shown no signs of recurrence.
Conclusion: In conclusion, even asymptomatic individuals should be highly regarded to prevent mistakes because the diagnostic process for this disease is complicated. The presence of metastatic cancer should be ruled out, and histology and immunohistochemistry should be used to confirm the diagnosis.
Introduction
Primary parotid squamous cell carcinoma (PPSCC) is a rare and aggressive malignant tumor with highly malignant potential, older age of onset, and more complex diagnostic procedures. Additionally, the incidence is increasing, and even with surgery and adjuvant radiotherapy (ART), the prognosis is dismal [1]. True PPSCC is very rare and accounts for 0.1–3.4% of all parotid malignancies [2]. According to some researchers, PPSCC can be identified if high-grade mucoepidermoid carcinoma (MEC) and metastatic parotid squamous cell carcinoma (SCC) are ruled out [3]. However, there are very few reports in the literature on diagnosing and treating PPSCC. Additionally, the majority of publications only involve a small number of individuals, perhaps because this disease is uncommon. In this case, the patient changed the treatment plan intraoperatively to re-expand the resection because of the difficult diagnosis and variable pathologic returns, which lengthened the procedure and raised the surgical risk. Due to these factors, this paper aims to raise awareness of PPSCC by presenting a case study and a review of the relevant scientific literature.
Case Report
A 62-year-old male patient presented with a painless mass in the left preauricular region for more than two months and was admitted with a left parotid tumor. There was no previous history of a mass in the same area or the neck. He had been treated at a local hospital before admission, and his diagnosis was unknown. On examination, the patient had a palpable mass in the left parotid gland, measuring about 3 cm by 2 cm by 2 cm, hard, inactive, adherent to the surrounding tissues, non-tender, and without symptoms of facial paralysis. There were multiple enlarged lymph nodes in the neck.
An enhanced computed tomography (CT) examination was performed after admission, and there was a soft tissue shadow next to the left masseter muscle, involving the masseter muscle and the left parotid gland, considering the possibility of a malignant occupational lesion of salivary gland origin. Enhanced MRI showed abnormal signals in the left masseter muscle and the left parotid gland, and an infectious lesion with abscess formation was considered possible. The patient also underwent fine-needle aspiration cytology (FNAC), and the puncture fluid was sent for pathologic examination. The pathology report showed that the tissue sent for examination showed more inflammatory cell infiltration and multinucleated cell aggregation, and small foci of moderate heterogeneous epithelial hyperplasia were seen. Immunohistochemical results: CK8/18 (–), P63 (+), Ki67 (+20%), S100 (–), SMA (–), CD163 (–), SOX (–). Special staining results: AB/PAS (–. The patient was admitted to the hospital for an infusion and anti-inflammatory treatment, and the self-conscious swelling became smaller. Preliminary Diagnosis: Consider lymphadenitis first, tumor second, and nature to be investigated.
On the 15th day after the patient was admitted to the hospital, “resection of parotid tumor” was performed under general anesthesia. The tumor was located on the deep lobe of the parotid gland, with no peripheral membrane, an unclear boundary, a hard texture, poor mobility, and the marginal mandibular branch of the facial nerve (FN) tightly adherent to the tumor. Partial excision of tumor tissue was sent intraoperatively for frozen pathology return (left parotid mass): consider inflammatory lesions. A “resection of parotid tumor + dissection of the FN” was performed, and after the tumor was removed, a second frozen pathology report was sent (left parotid mass): moderately differentiated SCC. “Extended resection of parotid malignant tumor + supraglottic lymphadenectomy” was performed. Intraoperative frozen (left parotid lymph node) was sent: no cancer metastasis was seen. Postoperative pathological findings: moderately differentiated SCC with no cancer metastasis in the lymph nodes. Immunohistochemical results: CK (+), CK7 (–), p40 (+), p63 (+), p53 (mutant+), and Ki67 (40%+).
In this case, the patient received the recommendation for postoperative ART, but it was postponed for personal reasons. There are no signs of recurrence and long-term follow-up needs to be further observed.
Discussion
Primary parotid squamous cell carcinoma is a malignant tumor that is uncommon, aggressive, and has a very negative prognosis. Squamous cell carcinoma makes up between 9% and 17% of parotid adenocarcinomas and between 0.1% and 3.4% of all parotid tumors [4]. The disease typically affects elderly people and has a brief duration and rapid progression. In most patients, the disease manifests as a painless neck mass, an infiltrative growth that is hard and adheres to surrounding tissues. It is simple to misdiagnose a patient’s illness, delaying treatment [5]. Our patient was 62 years old, had a smooth skin surface without any erosions or breakouts, and did not exhibit any symptoms like facial paralysis or pain. He had been seen by a local hospital without receiving a definitive diagnosis, and when he came to our hospital, he complained of a tumor that had shrunk. This led to the first diagnosis of lymphadenitis.
Primary parotid squamous cell carcinoma is rare and typically results from the metastasis of an extranodal original tumor. Right now, the diagnosis is one of exclusion. If the patient has a history of SCC of the head and neck, other distant sites, or contemporaneous SCC, salivary gland involvement is regarded as metastatic. When paired with a thorough clinical history and improved cross-sectional imaging, it is typically able to tell the difference between primary and metastatic parotid gland SCC [1],[2].
Magnetic resonance imaging should be the imaging method of the first choice. It is efficient in verifying suspected malignancy and can provide extensive anatomical information regarding the primary tumor’s location and its relationship to the FN and surrounding tissues [3],[4]. In addition to the well-known signs of parotid malignancy, such as poorly defined borders, large tumor size with noticeable invasiveness, irregular shape, infiltration of extra-parotid lymph nodes, and low to moderate signal intensity on T2-weighted images, relatively large tumors with central necrosis is a useful imaging feature of SCC originating in the parotid gland (Figure 1) [5],[6].
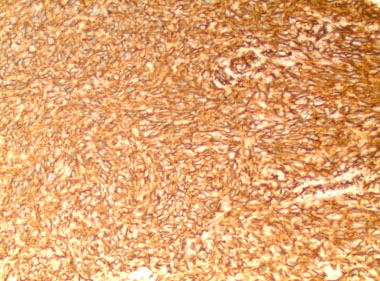
Furthermore, to accurately diagnose PPSCC, metastatic SCC of the parotid gland and high-grade MEC must be excluded [7]. High-grade MEC with a predominantly squamous component is very difficult to distinguish from SCC of the parotid gland with the aid of histopathologic examination, in contrast to SCC of the parotid gland, where low-grade and intermediate-grade MEC rarely cause diagnostic confusion. Also, MEC stain positive for intracellular mucin with PAS or mucicarmine stains which are absent in parotid SCC [8]. Almost all reported PPSCCs have been categorized as moderately highly differentiated and exhibit all the traditional histologic characteristics, as stated by Batsakis et al. for the creation of intracellular bridges, intracellular keratinization, keratin pearls, and a lack of mucin synthesis (Figure 2) [9].
In order to obtain a reliable histologic diagnosis, Mezi et al. [3] recommended a FNAC before surgery. Fine-needle aspiration cytology is widely used in the preoperative adjunctive diagnosis of head and neck tumors, but its diagnostic value for salivary gland tumors is controversial. It has been shown that the ability of FNA to differentiate between benign lesions and malignant tumors for all samples is 83%, for benign tumors 89%, and for malignant tumors 59% [10]. Therefore, FNA is more accurate in the diagnosis of benign parotid tumors. The accuracy of FNA depends on the quality of the sample material and the pathologist’s experience in evaluating it [11]. In this case, the result was false negative, with more inflammatory cell infiltration and multinucleated cell aggregates seen in the perforated tissue, and small foci of moderate anisotropic epithelial hyperplasia (Figure 3). False negatives may be the result of the needle localizing outside the target cells and failing to aspirate tissue from the lesion, necrosis within the tumor, hemorrhage, penetration into areas of cystic degeneration, or poor quality push slides [11],[12]. It can be highly helpful to the operator in developing a treatment plan as a first line of defense for the diagnosis of parotid malignancy, but some surgeons are less confident due to its relatively low sensitivity, false-negative, and false-positive outcomes [13],[14]. While intraoperative frozen sections are extremely reliable for detecting PPSCC, they are also crucial for excluding malignancy when the results of an FNAB are in doubt [15]. A basis for a clear intraoperative treatment plan is provided. Additionally, as PPSCC typically lacks any mucous cells, the application of specific stains (such as PAS reaction, Astra, or Alzian blue) is a useful supplementary diagnostic approach to distinguish MEC [8],[16].
The patient in our situation was a 62-year-old male. No other primary lesions were discovered during the preoperative clinical examination, enhanced CT of the head, neck, and chest, or enhanced MRI, and metastatic SCCs of the parotid gland were ruled out. In order to exclude high-grade MEC, the pathologic diagnosis of PAS (–) was used to corroborate the diagnosis of primary SCC of the parotid gland.
The aggressive nature of parotid SCC necessitates multimodal therapy, which includes surgical resection, neck surgery, adjuvant radiation, and/or chemotherapy [1]. The extent of initial surgery is critical to the success of treatment. Superficial parotidectomy combined with dissection of the FN is the recommended minimum extent of resection for primary SCC [17]. According to Xiao. [18] total parotidectomy is advised for patients who have primary or recurring SCC of the parotid gland. It is advised to leave the FN intact after parotidectomy if it has not been invaded. It is advised to remove the infiltrated facial nerve and its branches during parotidectomy if the FN is affected. Previous studies have advised standard elective cervical dissection in all patients with primary parotid carcinoma and clinically negative necks for cervical lymph nodes. For patients of primary or recurrent parotid SCC at stage cN0, selective cervical lymph node dissection is advised, while radical cervical lymph node dissection is advised for cases at stage cN1 + [17],[18],[19]. Furthermore, Lee et al. [20] concluded that postoperative radiation is thought to be necessary due to its most typical form of failure, localized regional recurrence. Within six weeks of surgery, Xiao [18] highly advise adjuvant radiation to the ipsilateral neck and parotid bed. 60–70 Gy should be the dose. The surgical bed should be included in the radiation field, along with the ipsilateral neck and parotid bed. Additionally, Mezi et al. [3] came to the conclusion that a standard dose of 100 mg/mq of cisplatin combined with radiochemotherapy should be given every three weeks to young patients with parotid SCC who have a good physical status score (PS) in order to lower the high risk of progression after surgical treatment. While Xiao [18] believe that chemotherapy/targeted therapy is only recommended for advanced and/or recurrent cases. For advanced and unresectable cases, chemotherapy/targeted therapy is only recommended as a palliative treatment, especially for those cases where surgery is not indicated. Finally, integration of nearby tissue and reconstruction to heal the tissue defect may be considered in cases with substantial resection of parotid SCC. For the repair of skin or mucosal abnormalities in the parotid region, adjacent flaps (Z-flap, gliding flap, or rhombic flap) are advised; anterolateral thigh flap (ALTF) or pectoralis major myocutaneous flap (PMMF) are suggested as alternatives. Reconstruction is only advised for muscle or bone abnormalities when there are negative margins.
Squamous cell carcinomas of the parotid gland have a poor prognosis, with a median patient survival of just 13–24 months and a previously reported five-year survival at around 50%. Additionally, even with surgery and ART, the prognosis is poor and the prevalence is rising [17],[18],[20],[21]. Black race, age 75 and older, tumor T3 and above, and high clinical stage were found to be significant predictors of poor prognosis in the study by Pfisterrell et al. [22]. Adjuvant radiation considerably decreased locoregional recurrence in patients overall, but it had no appreciable impact on survival rates [23]. The inclusion of chemotherapy to adjuvant therapy in patients with advanced salivary gland SCC may increase patients’ long-term survival [24].
In this case, after the intraoperative frozen pathology results were reported, the patient underwent “extended resection of parotid malignant tumor + dissection of the FN + supraglottic lymphadenectomy,” and in order to minimize recurrence, the patient is advised to undergo ART one month after the operation. The patient currently exhibits clear indicators of facial paralysis of the left lower lip and the elimination of the left frontal line without any indications of recurrence, but further research is needed to determine the long-term therapeutic benefit.


Conclusion
In conclusion, even asymptomatic individuals should be highly regarded to prevent mistakes because the diagnostic process for this disease is complicated and clinical features are crucial for the diagnosis. The presence of metastatic cancer should be ruled out, and histology and immunohistochemistry should be used to confirm the diagnosis. Patients should be taken into account for “extended resection of malignant tumors” and “selective neck dissection.” The preservation of the FN may be an option for patients whose tumors do not affect the FN, but this treatment carries a high risk of recurrence and distant metastases, and the prognosis is dismal, thus all patients should get postoperative adjuvant therapy. Therefore, postoperative adjuvant therapy ought to be given to all patients. This condition necessitates lifetime monitoring of patients.
REFERENCES
1.
Franzen A, Lieder A, Guenzel T, Buchali A. The heterogenicity of parotid gland squamous cell carcinoma: A study of 49 patients. In Vivo 2019;33(6):2001–6. [CrossRef]
[Pubmed]

2.
Edafe O, Hughes B, Tsirevelou P, Goswamy J, Kumar R. Understanding primary parotid squamous cell carcinoma – A systematic review. Surgeon 2020;18(1):44–8. [CrossRef]
[Pubmed]

3.
Mezi S, Pomati G, Botticelli A, et al. Primary squamous cell carcinoma of major salivary gland: “Sapienza Head and Neck Unit” clinical recommendations. Rare Tumors 2020;12:2036361320973526. [CrossRef]
[Pubmed]

4.
Bartels S, Talbot JM, DiTomasso J, et al. The relative value of fine-needle aspiration and imaging in the preoperative evaluation of parotid masses. Head Neck 2000;22(8):781–6. [CrossRef]
[Pubmed]

5.
Takahashi H, Kashiwagi N, Chikugo T, Nakanishi K, Tomita Y, Murakami T. Squamous cell carcinoma originating in the parotid gland: MRI features with histopathological correlation. Clin Radiol 2014;69(1):41–4. [CrossRef]
[Pubmed]

6.
Ban X, Hu H, Li Y, et al. Morphologic CT and MRI features of primary parotid squamous cell carcinoma and its predictive factors for differential diagnosis with mucoepidermoid carcinoma. Insights Imaging 2022;13(1):119. [CrossRef]
[Pubmed]

7.
Jo U, Song JS, Choi SH, Nam SY, Kim SY, Cho KJ. Primary squamous cell carcinoma of the salivary gland: Immunohistochemical analysis and comparison with metastatic squamous cell carcinoma. J Pathol Transl Med 2020;54(6):489–96. [CrossRef]
[Pubmed]

8.
Akhtar K, Ray PS, Sherwani R, Siddiqui S. Primary squamous cell carcinoma of the parotid gland: A rare entity. BMJ Case Rep 2013;2013:bcr2013009467. [CrossRef]
[Pubmed]

9.
Batsakis JG, McClatchey KD, Johns M, Regazi J. Primary squamous cell carcinoma of the parotid gland. Arch Otolaryngol 1976;102(6):355–7. [CrossRef]
[Pubmed]

10.
Boldes T, Hilly O, Alkan U, et al. Accuracy, predictability and prognostic implications of fine-needle aspiration biopsy for parotid gland tumours: A retrospective case series. Clin Otolaryngol 2021;46(5):1065–72. [CrossRef]
[Pubmed]

11.
Zbären P, Schär C, Hotz MA, Loosli H. Value of fine-needle aspiration cytology of parotid gland masses. Laryngoscope 2001;111(11 Pt 1):1989–92. [CrossRef]
[Pubmed]

12.
Zurrida S, Alasio L, Tradati N, Bartoli C, Chiesa F, Pilotti S. Fine-needle aspiration of parotid masses. Cancer 1993;72(8):2306–11. [CrossRef]
[Pubmed]

13.
Marzouki HZ, Altabsh MA, Albakrei MO, Al-Khatib TA, Merdad MA, Farsi NJ. Accuracy of preoperative fine needle aspiration in diagnosis of malignant parotid tumors. Saudi Med J 2017;38(10):1000–6. [CrossRef]
[Pubmed]

14.
Ayral M, Akil F, Yilmaz U, Toprak SF, Dedeoğlu S, Akdağ M. The diagnostic value of fine needle aspiration biopsy in parotid tumors. Indian J Otolaryngol Head Neck Surg 2022;74(Suppl 3):5856–60. [CrossRef]
[Pubmed]

15.
Arabi Mianroodi AA, Sigston EA, Vallance NA. Frozen section for parotid surgery: Should it become routine? ANZ J Surg 2006;76(8):736–9. [CrossRef]
[Pubmed]

16.
Seifert G, Donath K. Differential diagnosis of squamous epithelial carcinoma of the salivary glands. [Article in German]. Pathologe 1998;19(3):201–8. [CrossRef]
[Pubmed]

17.
Flynn MB, Maguire S, Martinez S, Tesmer T. Primary squamous cell carcinoma of the parotid gland: The importance of correct histological diagnosis. Ann Surg Oncol 1999;6(8):768–70. [CrossRef]
[Pubmed]

18.
Xiao M, Liu J, You Y, Yang X, Wang Y. Primary squamous cell carcinoma of the parotid gland: Clinicopathological characteristics, treatment, and prognosis. Int J Oral Maxillofac Surg 2021;50(2):151–7. [CrossRef]
[Pubmed]

19.
Zbären P, Schüpbach J, Nuyens M, Stauffer E. Elective neck dissection versus observation in primary parotid carcinoma. Otolaryngol Head Neck Surg 2005;132(3):387–91. [CrossRef]
[Pubmed]

20.
Lee S, Kim GE, Park CS, et al. Primary squamous cell carcinoma of the parotid gland. Am J Otolaryngol 2001;22(6):400–6. [CrossRef]
[Pubmed]

21.
Chen MM, Roman SA, Sosa JA, Judson BL. Prognostic factors for squamous cell cancer of the parotid gland: An analysis of 2104 patients. Head Neck 2015;37(1):1–7. [CrossRef]
[Pubmed]

22.
Pfisterer MJ, Vazquez A, Mady LJ, Khan MN, Baredes S, Eloy JA. Squamous cell carcinoma of the parotid gland: A population-based analysis of 2545 cases. Am J Otolaryngol 2014;35(4):469–75. [CrossRef]
[Pubmed]

23.
Renehan AG, Gleave EN, Slevin NJ, McGurk M. Clinico-pathological and treatment-related factors influencing survival in parotid cancer. Br J Cancer 1999;80(8):1296–300. [CrossRef]
[Pubmed]

24.
Cheraghlou S, Schettino A, Zogg CK, et al. Adjuvant chemotherapy is associated with improved survival for late-stage salivary squamous cell carcinoma. Laryngoscope 2019;129(4):883–9. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Jingli Zhao - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Xinrong Nan - Revising it critically for important intellectual content, Final approval of the version to be published
Chuhuan Zhou - Acquisition of data, Revising it critically for important intellectual content, Final approval of the version to be published
Nan Jiang - Analysis of data, Revising it critically for important intellectual content, Final approval of the version to be published
Liangliang Tian - Interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published
Data Availability StatementThe corresponding author is the guarantor of submission.
Consent For PublicationWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Competing InterestsAuthors declare no conflict of interest.
Copyright© 2024 Jingli Zhao et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.