|
Case Report
Pembrolizumab leading to complete resolution of non-small cell lung cancer and microsatellite instability stable colon adenocarcinoma; two birds one stone
1 Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555, USA
2 Department of Internal Medicine, University of Washington, Seattle, WA 98195, USA
3 Department of Pathology, University of Texas Medical Branch, Galveston, TX 77555, USA
4 Division of Oncology, University of Texas Medical Branch, Galveston, TX 77555, USA
5 Department of General Oncology, MD Anderson, Houston, TX 77030, USA
6 Department of Allergy and Immunology, University of Texas Medical Branch, Galveston, TX 77555, USA
Address correspondence to:
Pooja Bhakta
University of Texas Medical Branch, 300 Harborside Drive, Galveston, TX 77555,
USA
Message to Corresponding Author
Article ID: 100111Z10PB2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Bhakta P, Salazar L, Youssef A, Kendrick JC, Patel N, Willis M, Muthukumarana P, He J, Tripple J. Pembrolizumab leading to complete resolution of nonsmall cell lung cancer and microsatellite instability stable colon adenocarcinoma; two birds one stone. J Case Rep Images Oncology 2022;8(2):20–27.ABSTRACT
Introduction: Staging of non-small cell lung cancer is crucial in predicting patient prognosis and more importantly, determining cancer management. In patients without driver mutations, PD-L1 tumor proportion score evaluation becomes vital in dictating treatment, as immunotherapy can be recommended. These agents have been shown to lead to excellent outcomes, even in patients with late-stage disease.
Case Report: A 69-year-old male with a history of chronic obstructive pulmonary disease (COPD) presented with worsening dyspnea found to have lung collapse from a large hilar soft tissue mass causing obstruction of the left mainstem bronchus. After malignancy workup, the patient was diagnosed with non-small cell lung cancer clinically staged as IIIB. An incidental finding of microsatellite instability colon cancer was also found during workup. Pembrolizumab treatment was initiated and led to near resolution of both tumors.
Conclusion: Stage IIIB non-small cell lung cancer has an overall poor prognosis. Biomarker testing in our case prior to starting concurrent chemoradiation revealed the malignancy to have a 100% tumor proportion score for PD-L1, the fundamental reason why our patient’s treatment was successful. Based on our findings, we advocate for all patients with non-small cell lung cancer regardless of stage to undergo biomarker testing prior to therapy initiation. Furthermore, the resolution of PD-L1 negative microsatellite instability stable colon cancer after pembrolizumab therapy supports further investigation of the utility and mechanism of PD-1/PD-L1-based therapy in PD-L1 negative colon cancer.
Keywords: Colorectal cancer, Genotyping, Microsatellite instability, Non-small cell lung cancer, Pembrolizumab
Introduction
Lung cancer is the leading cause of cancer-related deaths in patients within the United Sates and remains the second most common cancer overall [1]. Increases in screening tests and decreased use of tobacco has reduced mortality rates by 23% in females and 48% in males; however, deaths from lung cancer remain high [1]. There are two major types of lung cancer—small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). In the past two decades, many advancements have been made in the treatment and management of NSCLC [2]. Stage IIIB NSCLC has an overall poor prognosis with 5–10% overall survival (OS) at five years [3]. Anti-PD-1 and PD-L1 antibodies block immune-checkpoint modulators boosting the immune system’s response against cancer cells and have shown excellent responses in NSCLC patients [4]. Colorectal cancer (CRC) arises from dysfunctional molecular pathways and includes microsatellite instability (MSI) tumors with a high degree of MSI have been known to respond to immunotherapy [5],[6],[7]. In this case report, we discuss a patient who achieved near complete resolution of NSCLC and complete resolution of MSI stable colon mass with single agent pembrolizumab.
Case Report
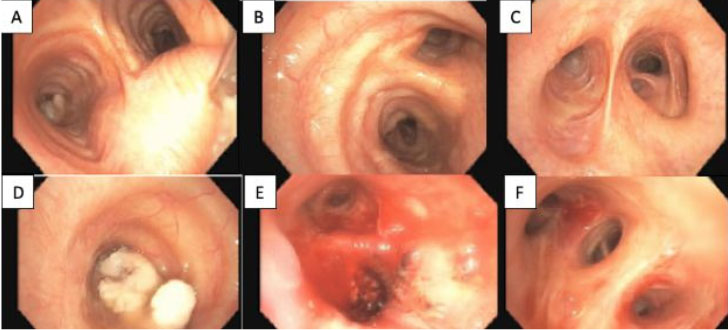
A 69-year-old male with 55 pack-year smoking history, COPD, hypertension, and hyperlipidemia presented to the emergency department with dyspnea. Initially he was found to be in acute hypoxic respiratory failure (AHRF) from left lung atelectasis requiring hospital admission for further workup. Computed tomography (CT) chest revealed a large hilar soft tissue mass (3.2×2.6 cm) which led to total obstruction of the left mainstem bronchus and consequent left lung collapse. Computed tomography imaging also revealed significant hilar and mediastinal lymphadenopathy (1.1 cm). The patient underwent flexible bronchoscopy with tumor debulking of the left mainstem bronchus, left upper lobe, and left lower lobe (Figure 1). Pathology revealed a poorly differentiated malignancy arranged in sheets with areas of necrosis. No gland formation or squamous differentiation was seen. Tumor cells showed enlarged nuclei, vesicular chromatin, and prominent nucleoli. Some areas showed discohesive pleomorphic cells with abundant mitosis. Immunohistochemical stains and special stains were performed to further characterize the malignant neoplasm. Tumor cells were diffusely positive for cytokeratin 7. Negative stains included lung adenocarcinoma markers (TTF-1, Napsin A, mucicarmine), lower gastrointestinal markers (CK20, CDX2, CDH17), squamous cell carcinoma markers (p40), and neuroendocrine markers (synaptophysin, chromogranin). PD-L1 biomarker testing revealed 100% tumor proportion score (TPS) (Table 1). Genotypic analysis did not reveal ALK, BRAF, EGFR, ERBB2, KRAS, RET, or ROS1 driver mutation and was equivocal for CDK4, CCND2, CDK6, EPHB4, and FGF23 driver mutations (Table 1). Because a total excision of the tumor was not obtainable, a diagnosis of poorly differentiated NSCLC not otherwise specified (NOS) was made. Given the presence of a solitary large hilar lung mass, these findings were consistent with primary non-small cell lung carcinoma (NSCLC) (Figure 2).
Further malignancy workup including CT abdomen and pelvis, magnetic resonance imaging (MRI) brain, and positron emission tomography (PET) was performed. Computed tomography abdomen imaging revealed a segment of mural thickening in the transverse colon. Positron emission tomography revealed hypermetabolic activity in the ill-defined left infrahilar mass, mediastinal lymph nodes, and paraspinal lymph nodes, but was not indicative of any underlying metastatic disease. A colonoscopy was then performed and revealed a 5 mm elevated non-polypoid lesion in the transverse colon (Figure 3). A core biopsy was obtained; however, endoscopic mucosal resection was not performed due to the perceived increased risks associated with the more complex non-pedunculated lesion removal weighed against the minimal benefit in this patient with advanced NSCLC. Biopsy pathology results identified poorly differentiated adenocarcinoma with microsatellite instability (MSI) stable and mucosal involvement (Figure 4 and Figure 5). The colon tumor was determined to be a primary lesion given the rarity of metastatic lung disease to the colon and that mucosal involvement was more consistent with primary disease. The patient’s NSCLC was consequently clinically staged as IIIB (cT2 cN3 cM0) based on imaging findings.
Monotherapy with pembrolizumab was chosen due to the absence of driver mutations and a 100% TPS. The patient underwent treatment with pembrolizumab every 42 days with palliative and life prolonging intent. After four cycles, there was significant reduction in the left hilar mass (2.5×1.7×2.9 cm) and mediastinal lymphadenopathy size (0.9 cm).
Treatment course was complicated by pneumonia development and COPD exacerbation requiring hospital admission, subsequently leading to delays in treatment. Interim imaging during hospitalization also revealed an apparent reduction of previously observed colonic thickening, believed to be due to positive response to pembrolizumab therapy. After six cycles of pembrolizumab therapy, CT imaging showed near resolution of the left hilar soft tissue mass (1.6×0.7 cm). A repeat colonoscopy one year later showed no mass at the marked site within the transverse colon (Figure 3). Our patient has since had approximately 11 cycles of immunotherapy with pembrolizumab with near complete resolution of his NSCLC without visualization of the mass on follow-up imaging.
Discussion
Cytotoxic T lymphocytes are not only involved in protection against infections but also in the surveillance of neoplasms. The immune inflammatory response to foreign or neoplastic antigen is regulated by several checkpoints of necessary co-stimulatory and inhibitory signals involving receptor-ligand interactions [8]. Cytotoxic T-lymphocyte (CTL) activation requires the interaction between the antigen-presenting cell (APC) major histocompatibility complex (MHC) molecules and the T-cell receptor (TCR), as well as costimulatory interaction between CTL receptor CD28 and APC ligand B7 [8]. Although there are several known inhibitory interactions between CTLs and APCs that modulate the stimulatory response, the most widely studied are the CTL receptor programmed death 1 (PD-1), APC ligand programmed death ligand 1 (PD-L1), and CTL receptor CTL associated protein 4 (CTLA-4) [8],[9],[10]. These inhibitory signals are utilized and variably expressed by neoplastic cells to evade the immune system [9]. After prolonged antigenic stimulation via inhibitory signals between APCs and CTLs, CTLs experience an “exhaustion” state in which they are not able to identify and eliminate neoplastic cells [10],[11]. As such, the study of these signals has been invaluable in cancer immunotherapy with the advent of immune checkpoint inhibitor (ICI) antibodies that target the PD-1, PD-L1, and CTLA-4 molecules with the goal of reinvigorating the immune response [8],[10].
Pembrolizumab is a humanized monoclonal IgG antibody that specifically binds to PD-1 on T-cells [12]. Neoplastic cell oncogenetics may increase expression of PD-L1 to improve tumor cell survival, thus forming the basis of utilizing anti-PD-1 therapy to counter neoplastic immune system evasion [9],[10]. Common adverse events (AEs) after pembrolizumab therapy include fatigue, pruritus, hypothyroidism, diarrhea, and nausea [13]. Serious AEs are often hematologic in nature and include anemia, leukopenia, thrombocytopenia, and rarely serious AEs such as cytokine release syndrome and secondary hemophagocytic lymphohistiocytosis [13]. Pembrolizumab therapy specifically has been studied in over 20 tumor types, not limited to melanoma, non-small cell lung cancer (NSCLC), advanced Merkel cell carcinoma, mismatch repair deficient cancers, and Hodgkin lymphoma [11],[14],[15]. Previous studies examining PD-1/PD-L1 ICIs have produced encouraging clinical responses in many cancers and have emphasized the importance of cancer differentiation by tumor biomarker status [16]. Outcomes of these studies suggest that patients with >50% PD-L1 positive tumor expression have overall better responses and outcomes, as compared with patients with 1–49% and <1% PD-L1 expression [14],[17].
Non-small cell lung cancer is a group of heterogeneous tumors with varying prognoses depending on molecular features, subtype, tumor size, and nodal status. However, prognosis is typically poor, and rates of treatment failure are high, especially in locally advanced or distant metastatic disease [2],[18],[19]. For stage IIIB locally advanced NSCLC, 5-year OS ranges from 7% to 9% [18]. However, treatment options have broadened and diverged from traditional therapy with radiation and platinum-based regimens over the last decade [2],[18],[20]. The identification of gene alterations (i.e., driver mutations) via molecular testing and genotyping has changed the landscape of management and prognosis for NSCLC [2]. Patients with identified driver gene mutations can then be given targeted tyrosine kinase inhibitor (TKI) therapies (e.g., KRAS, EGFR, ALK) [2],[21]. Patients without driver mutations, especially EGFR and ALK, should be evaluated for PD-L1 expression represented by the tumor proportion score (TPS%) [22]. In patients with a TPS > 50%, non-squamous cell carcinoma (SCC) subtype, and performance status 0–1, single agent therapy with pembrolizumab is recommended [22],[23],[24].
Non-small cell lung cancer has been identified among tumors as having some of the greatest number of protein-altering mutations [21]. As such, the high rate of mutation has allowed for the evolution of previous standards of care to the development of biomolecular marker and driver mutation directed treatments [4],[21],[23],[25]. Commonly tested gene mutations and biomarkers in NSCLC include p53, KRAS, MSI, PD-L1, p16 methylation, EGFR, ALK, and ROS1 as they are frequently mutated in these patients [21],[23],[26],[27]. However, genetic testing in locally advanced NSCLC has not been established as the standard of care [28]. The TPS% is becoming increasingly valuable as the therapeutic potential for PD-L1/PD-1 directed therapy is explored [4],[18],[25].
In patients with advanced NSCLC and a TPS > 50%, first-line therapy with pembrolizumab therapy was shown to be more effective than platinum chemotherapy (PFS 10.3 vs 6.0 months respectively) [23]. PD-L1 expression and the correlation to treatment response and prognosis is still an active area of study; however, analysis by Nadal et al. revealed an increased response rate in patients with higher PD-L1 expression versus lower expression rates [29]. Khoja et al. demonstrated that patients with NSCLC and PD-L1 expression ≥50% had higher overall response rate (ORR), longer progression free survival (PFS), and OS compared to patients with <50% PD-L1 expression [14]. Analysis of the Keynote-001 study, which was a phase 1 trial, evaluated pembrolizumab monotherapy in advanced NSCLC in treatment naïve and non-treatment naïve patients and concluded that pembrolizumab monotherapy provided durable antitumor activity and had higher 5-year OS rates [17]. The Keynote-001 study also demonstrated that patients with PD-L1 TPS ≥ 50% had a 5-year OS rate exceeding 25% [17]. In a pooled analysis by Nosaki et al. of patients ≥75 years of age with treatment naïve and non-treatment naïve advanced NSCLC who were PD-L1 positive from the Keynote-010, Keynote-024, and Keynote-042 studies, demonstrated that pembrolizumab improved OS compared with chemotherapy [30].
An additional consideration for immunotherapy that exists within the realm of stage III NSCLC is durvalumab, an IgG kappa monoclonal antibody that blocks PD-L1 from binding to PD-1 and CD80. As evidenced by the PACIFIC trial, this therapy is indicated for consolidative treatment following chemoradiotherapy in unresectable stage III NSCLC that has not progressed. Overall survival data at four years in the durvalumab arm was 47.5 months versus 29.1 months in the placebo arm, and progression-free survival at four years was estimated at 35.3% in the durvalumab arm versus 19.5% in the placebo arm. Overall survival data with durvalumab demonstrated a benefit in patients with PD-L1 expression greater than 1%. Progression-free survival data with durvalumab demonstrated a benefit in patients across all PD-L1 subgroups [29]. The PACIFIC trial has become a landmark study that further solidified the role of ICI therapy in locally advanced unresectable NSCLC regardless of the degree of PD-L1 expression.
Colorectal cancer (CRC) has been identified to primarily arise from dysfunctional molecular pathways resulting from chromosomal instability, mismatch repair, and hypermethylation [5]. Deficient mismatch repair (dMMR) systems result in the accumulation of errors generating microsatellite instability (MSI) and accounts for approximately 15% of sporadic CRC [5],[6]. Among gastrointestinal malignancies, CRC has the highest prevalence of MSI-associated tumors [31]. Tumors with high degrees of MSI (MSI-H) are associated with high tumor mutation burdens (TMB), measurable through evaluation of plasma TMB (pTMB) [7]. As a result of high TMB, MSI-H tumors have highly productive neoantigen presentation and are consequently infiltrated by T-cells [7]. The high degree of neoantigen presentation and T-cell infiltration (TCI), measurable as the immunoscore (IS), make MSI-H tumors effective targets of immunotherapy (e.g., PD-L1, PD-1, CTLA-4 antibodies) [7]. A study by Le et al. evaluated the benefit of single-agent pembrolizumab in the treatment of dMMR metastatic CRC (mCRC), MMR-proficient (pMMR) mCRC, and dMMR non-CRC [32]. They demonstrated that MMR status predicted clinical benefit of pembrolizumab therapy with greater benefit in dMMR tumors [32]. Evaluation of combination ipilimumab/nivolumab (anti-CTL-4 and anti-PD-1 respectively) therapy in MSI-H CRC was performed by Overman et al. [33]. Their study revealed that ipilimumab/nivolumab dual therapy was effective and was not dependent on PD-L1 expression [33].
However, only 4% of mCRC are MSI-H/dMMR, necessitating increased need for the development of effective treatments for microsatellite stable (MSS)/pMMR tumors [34]. Microsatellite stable CRC tumors are purported to be less responsive to ICI therapy because of low TMB and consequent low neoantigen presentation and TCI; however, the underlying mechanisms behind differing responses between MSS and MSI-H tumors are still under study [32].[35]. Although the role of immunotherapy in MSS CRC is still an area of active development, a recent study by Chalabi et al. evaluated neoadjuvant ICI therapy with ipilimumab/nivolumab in early stage pMMR and dMMR colon cancer (CC). They showed a proficient response to ICI therapy in both dMMR and pMMR CC after a single dose of ipilimumab and two doses of nivolumab, with 100% and 27% pathologic responses respectively [35]. The only notable biomarker that was correlated to pathologic response in pMMR tumors was baseline presence of CD8 and PD-1 T-cells (CD8+PD-1+ TCI) [35]. Furthermore, they noted that there were significant increases in post-treatment TCI in both pMMR responders and non-responders, which may play a valuable role in future studies on how post-ICI treatment in early-stage CRC potentiates further treatment [35]. These significant findings of ICI-responsive pMMR CC, baseline immune cell infiltration, and pre- and post-treatment TCI comparisons opens promising new avenues for the usage of ICI therapy in pMMR/MSS CRC and the importance of tumor genome sequencing to guide therapy.
Conclusion
Recent studies demonstrate strong evidence that pembrolizumab monotherapy is an efficacious and effective treatment for locally advanced NSCLC, as used in our patient. Although genotyping and molecular testing is not currently standard of care for locally advanced stage III NSCLC, we recommend this approach prior to therapy initiation in all patients. In patients without driver mutations, pembrolizumab with or without chemotherapy, can be a highly valuable treatment consideration, especially in patients with PD-L1 expression ≥50%. Furthermore, PD-L1 expression testing can be performed quickly and allow for rapid initiation of treatment, as opposed to molecular testing and genotyping. Similarly, genomic testing for MSI status should be performed in early-stage CRC as a means of guiding therapy. Early-stage neoadjuvant ICI therapy in MSS/pMMR CRC is an evolving field of study but current evidence shows immunotherapy is a promising therapy consideration in these patients.
REFERENCES
1.
Alexander M, Kim SY, Cheng H. Update 2020: Management of non-small cell lung cancer. Lung 2020;198(6):897–907. [CrossRef]
[Pubmed]

2.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553(7689):446–54. [CrossRef]
[Pubmed]

3.
Burdett SS, Stewart LA, Rydzewska L. Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev 2007(3):CD006157. [CrossRef]
[Pubmed]

4.
Patel SA, Weiss J. Advances in the treatment of nonsmall cell lung cancer: Immunotherapy. Clin Chest Med 2020;41(2):237–47. [CrossRef]
[Pubmed]

5.
6.
Cappell MS. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North Am 2008;37(1):1–24, v. [CrossRef]
[Pubmed]

7.
Motta R, Cabezas-Camarero S, Torres-Mattos C, et al. Immunotherapy in microsatellite instability metastatic colorectal cancer: Current status and future perspectives. J Clin Transl Res 2021;7(4):511–22.
[Pubmed]

8.
9.
Dobosz P, Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol 2019;10:2965. [CrossRef]
[Pubmed]

10.
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol 2020;17(8):807–21. [CrossRef]
[Pubmed]

11.
Kwok G, Yau TCC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother 2016;12(11):2777–89. [CrossRef]
[Pubmed]

12.
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369(2):134–44. [CrossRef]
[Pubmed]

13.
Mo DC, Luo PH, Huang SX, Wang HL, Huang JF. Safety and efficacy of pembrolizumab plus lenvatinib versus pembrolizumab and lenvatinib monotherapies in cancers: A systematic review. Int Immunopharmacol 2021;91:107281. [CrossRef]
[Pubmed]

14.
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann Oncol 2017;28(10):2377–85. [CrossRef]
[Pubmed]

15.
Nasser NJ, Gorenberg M, Agbarya A. First line immunotherapy for non-small cell lung cancer. Pharmaceuticals (Basel) 2020;13(11):373. [CrossRef]
[Pubmed]

16.
Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer 2018;6(1):35. [CrossRef]
[Pubmed]

17.
Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol 2019;37(28):2518–27. [CrossRef]
[Pubmed]

18.
Huber RM, De Ruysscher D, Hoffmann H, Reu S, Tufman A. Interdisciplinary multimodality management of stage III non-small cell lung cancer. Eur Respir Rev 2019;28(152):190024. [CrossRef]
[Pubmed]

19.
Evison M, AstraZeneca UK Limited. The current treatment landscape in the UK for stage III NSCLC. Br J Cancer 2020;123(Suppl 1):3–9. [CrossRef]
[Pubmed]

20.
Cheema PK, Rothenstein J, Melosky B, Brade A, Hirsh V. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr Oncol 2019;26(1):37–42. [CrossRef]
[Pubmed]

21.
Wadowska K, Bil-Lula I, Trembecki ?, ?liwi?ska-Mosso? M. Genetic markers in lung cancer diagnosis: A review. Int J Mol Sci 2020;21(13):4569. [CrossRef]
[Pubmed]

22.
Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2021;39(9):1040–91. [CrossRef]
[Pubmed]

23.
Gregg JP, Li T, Yoneda KY. Molecular testing strategies in non-small cell lung cancer: Optimizing the diagnostic journey. Transl Lung Cancer Res 2019;8(3):286–301. [CrossRef]
[Pubmed]

24.
Dietel M, Savelov N, Salanova R, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: The global, multicenter EXPRESS study. Lung Cancer 2019;134:174–9. [CrossRef]
[Pubmed]

25.
Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: Non-small cell lung cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19(3):254–66. [CrossRef]
[Pubmed]

26.
Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev Oncol Hematol 2021;157:103194. [CrossRef]
[Pubmed]

27.
Pikor LA, Ramnarine VR, Lam S, Lam WL. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82(2):179–89. [CrossRef]
[Pubmed]

28.
Huber RM, Kauffmann-Guerrero D, Hoffmann H, Flentje M. New developments in locally advanced nonsmall cell lung cancer. Eur Respir Rev 2021;30(160):200227. [CrossRef]
[Pubmed]

29.
Nadal E, Massuti B, Dómine M, García-Campelo R, Cobo M, Felip E. Immunotherapy with checkpoint inhibitors in non-small cell lung cancer: Insights from long-term survivors. Cancer Immunol Immunother 2019;68(3):341–52. [CrossRef]
[Pubmed]

30.
Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019;135:188–95. [CrossRef]
[Pubmed]

31.
Huynh JC, Schwab E, Ji J, et al. Recent advances in targeted therapies for advanced gastrointestinal malignancies. Cancers (Basel) 2020;12(5):1168. [CrossRef]
[Pubmed]

32.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372(26):2509–20. [CrossRef]
[Pubmed]

33.
Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 2018;36(8):773–9. [CrossRef]
[Pubmed]

34.
Das S, Ciombor KK, Haraldsdottir S, Goldberg RM. Promising new agents for colorectal cancer. Curr Treat Options Oncol 2018;19(6):29. [CrossRef]
[Pubmed]

35.
Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26(4):566–76. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Pooja Bhakta - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Leonardo Salazar - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ayman Youssef - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jamie C Kendrick - Acquisition of data, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nekita Patel - Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Maurice Willis - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Palawinnage Muthukumarana - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jing He - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Julia W Tripple - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Pooja Bhakta et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.